T919 Oxidation and Reduction Past Paper Questions
... Both zinc and tin are used to coat iron to prevent it from rusting. Once the surface is scratched, oxygen and water containing dissolved ions come into contact with the iron and the coating metal. ...
... Both zinc and tin are used to coat iron to prevent it from rusting. Once the surface is scratched, oxygen and water containing dissolved ions come into contact with the iron and the coating metal. ...
Chem Stoichiometry Study Guide
... 12. Identify the limiting and excess reactants when 1.00 g of zinc reacts with 150 mL of 0.250M Pb(NO 3)2. How many grams of lead are formed in this single replacement ...
... 12. Identify the limiting and excess reactants when 1.00 g of zinc reacts with 150 mL of 0.250M Pb(NO 3)2. How many grams of lead are formed in this single replacement ...
study packet for chapter 5
... B) water freezing C) boiling soup D) Hydrochloric acid and barium hydroxide are mixed at 25 °C: the temperature increases. E) Both A and C 7) Which one of the following is an exothermic process? A) ice melting B) water evaporating C) boiling soup D) condensation of water vapor E) Ammonium thiocyanat ...
... B) water freezing C) boiling soup D) Hydrochloric acid and barium hydroxide are mixed at 25 °C: the temperature increases. E) Both A and C 7) Which one of the following is an exothermic process? A) ice melting B) water evaporating C) boiling soup D) condensation of water vapor E) Ammonium thiocyanat ...
SYSTEMATIC NOMENCLATURE OF COORDINATION COMPOUNDS
... 4. When several ligands of a particular kind are present, use the Greek prefixes di-, tri-, tetra-, penta-, and hexa. Example: The ligands in [Pd(Cl)6]2- ion are hexachloro The ligands in [Co(NH3)4Cl2]+ are "tetraamminedichloro." (remember alphabetical order for the name of ligands and not their pr ...
... 4. When several ligands of a particular kind are present, use the Greek prefixes di-, tri-, tetra-, penta-, and hexa. Example: The ligands in [Pd(Cl)6]2- ion are hexachloro The ligands in [Co(NH3)4Cl2]+ are "tetraamminedichloro." (remember alphabetical order for the name of ligands and not their pr ...
1.4 Alcohols, Ethers, and Thiols
... • The IUPAC method is to add the suffix –oxy to the smaller hydrocarbon group that is bonded to the larger alkane group • A number may be required to indicate the carbon atom that the oxygen is attached to on the longer chain • A common naming system uses the names of the two hydrocarbon groups foll ...
... • The IUPAC method is to add the suffix –oxy to the smaller hydrocarbon group that is bonded to the larger alkane group • A number may be required to indicate the carbon atom that the oxygen is attached to on the longer chain • A common naming system uses the names of the two hydrocarbon groups foll ...
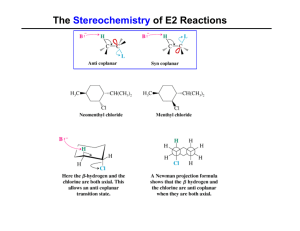
The Stereochemistry of E2 Reactions
... Dehydration of Alcohols: E1 Mechanism Rate Limiting Step ...
... Dehydration of Alcohols: E1 Mechanism Rate Limiting Step ...
Reduction of Camphor to Borneol
... showing the expected stereochemistry of the products. Label the products as having been formed from exo approach or endo approach. 2. How might the geometry of the product change (OH in an endo or exo position?) if all the methyl groups of camphor were replaced with H? 3. The reduction mechanism is ...
... showing the expected stereochemistry of the products. Label the products as having been formed from exo approach or endo approach. 2. How might the geometry of the product change (OH in an endo or exo position?) if all the methyl groups of camphor were replaced with H? 3. The reduction mechanism is ...
Highly Enantioselective Cyclocarbonylation of Allylic
... identify CH2Cl2 under a CO/H2 atmosphere (total pressure ) 800 psi, ratio of CO/H2 ) 1:1) at 80 °C with 1 mol % of Pd-catalyst(Table 1). The Pd-BICP catalyst was prepared in situ from Pd2(dba)3 and (R,R)-BICP. Under these conditions, cyclocarbonylation of 1a gave product 2a in 95%ee (entry 1). When ...
... identify CH2Cl2 under a CO/H2 atmosphere (total pressure ) 800 psi, ratio of CO/H2 ) 1:1) at 80 °C with 1 mol % of Pd-catalyst(Table 1). The Pd-BICP catalyst was prepared in situ from Pd2(dba)3 and (R,R)-BICP. Under these conditions, cyclocarbonylation of 1a gave product 2a in 95%ee (entry 1). When ...
Carbon
... is bonded to the carbon skeleton; two oxygens carry negative charges. The phosphate group (—OPO32–, abbreviated P ) is an ionized form of a phosphoric acid group (—OPO3H2; note the two ...
... is bonded to the carbon skeleton; two oxygens carry negative charges. The phosphate group (—OPO32–, abbreviated P ) is an ionized form of a phosphoric acid group (—OPO3H2; note the two ...
Transition Metals
... The 3d orbitals are not as important for bonding as are the 4s and 4p, but the details of what happens to the 3d orbitals determine the properties of transition metal complexes. ...
... The 3d orbitals are not as important for bonding as are the 4s and 4p, but the details of what happens to the 3d orbitals determine the properties of transition metal complexes. ...
2008 local exam - American Chemical Society
... (A) The initial precipitate will contain CaF2 only. (B) The initial precipitate will contain MgF2 only. (C) The initial precipitate will contain both CaF2 and MgF2 with more CaF2. (D) The initial precipitate will contain both CaF2 and MgF2 with more MgF2. 37. Which range includes the average oxidati ...
... (A) The initial precipitate will contain CaF2 only. (B) The initial precipitate will contain MgF2 only. (C) The initial precipitate will contain both CaF2 and MgF2 with more CaF2. (D) The initial precipitate will contain both CaF2 and MgF2 with more MgF2. 37. Which range includes the average oxidati ...
Chapter 12: Introduction to Organic Chemistry
... 1. If less than 2n+2 H’s, then unsaturated or cyclic B. Nomenclature 1. prefix cyclo- in front of parent chain 2. substituents numbered around ring a. such as to give lowest numbers b. 1,3 not 1,4 3. when two different groups present a. first alphabetically gets lowest number b. ignore di, tri, etc. ...
... 1. If less than 2n+2 H’s, then unsaturated or cyclic B. Nomenclature 1. prefix cyclo- in front of parent chain 2. substituents numbered around ring a. such as to give lowest numbers b. 1,3 not 1,4 3. when two different groups present a. first alphabetically gets lowest number b. ignore di, tri, etc. ...
Compounds Containing a C=O (Carbonyl) Group
... Triacylglycerols contain three ester groups, each having a long carbon chain (abbreviated as R, R', and R") bonded to the carbonyl group. Triacylglycerols are lipids; that is, they are water-insoluble organic compounds found in biological systems. Animal fats and vegetable oils are composed of triac ...
... Triacylglycerols contain three ester groups, each having a long carbon chain (abbreviated as R, R', and R") bonded to the carbonyl group. Triacylglycerols are lipids; that is, they are water-insoluble organic compounds found in biological systems. Animal fats and vegetable oils are composed of triac ...
Accepted Manuscript
... properties of niobium oxide catalysts. Niobia-doped ceria materials have shown agood tolerance to carbon deposition [9] and excellentproperties as solid oxide fuel cell (SOFC) anodes[12]. However, more research is necessary in order to extent the improved performance of these systems for other appli ...
... properties of niobium oxide catalysts. Niobia-doped ceria materials have shown agood tolerance to carbon deposition [9] and excellentproperties as solid oxide fuel cell (SOFC) anodes[12]. However, more research is necessary in order to extent the improved performance of these systems for other appli ...
Chemical Equations PowerPoint
... c) Indications of a chemical reaction (chemical change): – evolution of heat and/or light – production of a gas (often seen as bubbles) – color change – formation of a precipitate when two solutions are mixed (Precipitate = a solid that separates from a solution) ...
... c) Indications of a chemical reaction (chemical change): – evolution of heat and/or light – production of a gas (often seen as bubbles) – color change – formation of a precipitate when two solutions are mixed (Precipitate = a solid that separates from a solution) ...
Information Regarding Prof
... There was development of an effective way to construct the C-7a quaternary chiral center of anisatin via an Eschenmoser- Claisen rearrangement. Also, a formal synthesis of (+/-)8-deoxyanisatin is completed due to conversion of resultant amide to Kende’s epsilonlactose intermediate 3 in four steps. 1 ...
... There was development of an effective way to construct the C-7a quaternary chiral center of anisatin via an Eschenmoser- Claisen rearrangement. Also, a formal synthesis of (+/-)8-deoxyanisatin is completed due to conversion of resultant amide to Kende’s epsilonlactose intermediate 3 in four steps. 1 ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.