* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Compounds Containing a C=O (Carbonyl) Group
Bottromycin wikipedia , lookup
Metal carbonyl wikipedia , lookup
Physical organic chemistry wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Aldol reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
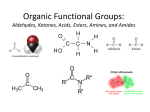
ORGANIC CHEMISTRY Dr. Serkan SAYINER [email protected] Compounds Containing a C=O (Carbonyl) Group Aldehydes, Ketones, Carboxylic acids, Esters, and Amides Compounds Containing a C=O Group Many different kinds of compounds contain a carbon– oxygen double bond (C=O, carbonyl group). Carbonyl compounds include aldehydes, ketones, carboxylic acids, esters, and amides. The type of atom bonded to the carbonyl carbon— hydrogen, carbon, or a heteroatom—determines the specific class of carbonyl compound. Compounds Containing a C=O Group Take special note of the condensed structures used to draw aldehydes, carboxylic acids, and esters. An aldehyde has a hydrogen atom bonded directly to the carbonyl carbon. Compounds Containing a C=O Group A carboxylic acid contains an OH group bonded directly to the carbonyl carbon. An ester contains an OR group bonded directly to the carbonyl carbon. Asetaldehit Aseton Asetik asit Metil asetat Asetamid Aldehydes and Ketones Naming Aldehydes, Naming Ketones, Physical Properties, Interesting Aldehydes and Ketones, Preparation of Aldehydes and Ketones, Reactions of Aldehydes and Ketones (Addition of 1° Amines, Addition of 2° Amines, Addition of H2O—Hydration, Addition of Alcohols—Acetal Formation, Acetals as Protecting Groups, Cyclic Hemiacetals, Oxidation of Aldehydes) Dr. Serkan SAYINER [email protected] Aldehydes and Ketones Aldehydes (RCHO) and Ketones (RCOR or R2CO) are two families of compounds that contain a carbonyl group. Two structural features dominate the properties and chemistry of the carbonyl group. • The carbonyl carbon atom is trigonal planar, and all bond angles are 120°. • Since oxygen is more electronegative than carbon, a carbonyl group is polar. • The carbonyl carbon is electron poor (𝛅+) and the oxygen is electron rich (𝛅–). Naming Aldehydes In IUPAC nomenclature, aldehydes are identified by the suffix - al. To name an aldehyde using the IUPAC system: 1. Find the longest chain containing the CHO group, and change the -e ending of the parent alkane to the suffix -al. 2. Number the chain or ring to put the CHO group at C1, but omit this number from the name. 3. Apply all of the other usual rules of nomenclature. 4. Simple aldehydes have common names that are virtually always used instead of their IUPAC names. Common names all contain the suffix aldehyde. Naming Ketones In the IUPAC system, ketones are identified by the suffix -one. To name an acyclic ketone using IUPAC rules: 1. Find the longest chain containing the carbonyl group, and change the -e ending of the parent alkane to the suffix -one. 2. Number the carbon chain to give the carbonyl carbon the lower number. Apply all of the other usual rules of nomenclature. Naming Ketones With cyclic ketones, numbering always begins at the carbonyl carbon, but the “1” is usually omitted from the name. The ring is then numbered clockwise or counterclockwise to give the first substituent the lower number. Most common names for ketones are formed by naming both alkyl groups on the carbonyl carbon, arranging them alphabetically, and adding the word ketone. Three simple ketones have widely used common names. Physical Properties Because aldehydes and ketones have a polar carbonyl group, they are polar molecules with stronger intermolecular forces than the hydrocarbons. • Aldehydes and ketones exhibit dipole–dipole interactions because of their polar carbonyl group. Since they have no O—H bond, two molecules of RCHO or RCOR are incapable of intermolecular hydrogen bonding, giving them weaker intermolecular forces than alcohols. Physical Properties As a result: • Aldehydes and ketones have higher boiling points than hydrocarbons of comparable size. • Aldehydes and ketones have lower boiling points than alcohols of comparable size. Physical Properties Based on the general rule governing solubility (i.e., “like dissolves like”), aldehydes and ketones are soluble in organic solvents. Moreover, because aldehydes and ketones contain an oxygen atom with an available lone pair, they can intermolecular hydrogen bond to water. Physical Properties Low molecular weight aldehydes and ketones (those having less than six carbons) are soluble in both organic solvents and water. Higher molecular weight aldehydes and ketones (those having six carbons or more) are soluble in organic solvents, but insoluble in water. Acetone and progesterone are two ketones that occur naturally in the human and animal body. Interesting Aldehydes and Ketones Many aldehydes with characteristic odors occur in nature. • Cinnamaldehyde, the major component of cinnamon bark, is a common flavoring agent. • Vanillin is the primary component of the extract of the vanilla bean. Because natural sources cannot meet the high demand, most vanilla flavoring agents are made synthetically from starting materials derived from petroleum. • Geranial has the lemony odor characteristic of lemon grass. Geranial is used in perfumery and as starting material for synthesizing vitamin A. • Citronellal gives the distinctive lemon odor to citronella candles, commonly used to repel mosquitoes. Interesting Aldehydes and Ketones Because it is a starting material for the synthesis of many resins and plastics, billions of pounds of formaldehyde are produced annually in the United States by the oxidation of methanol (CH3OH). Formaldehyde is also sold as a 37% aqueous solution called formalin, which has been used as a disinfectant, antiseptic, and preservative for biological specimens. Formaldehyde, a product of the incomplete combustion of coal and other fossil fuels, is partly responsible for the irritation caused by smoggy air. Interesting Aldehydes and Ketones Acetone [(CH3)2C=O, the simplest ketone] is produced naturally in cells during the breakdown of fatty acids. In diabetes, a disease where normal metabolic processes are altered because of the inadequate secretion of insulin, individuals often have unusually high levels of acetone in the bloodstream. The characteristic odor of acetone can be detected on the breath of diabetic patients when their disease is poorly controlled. Interesting Aldehydes and Ketones Ketones play an important role in the tanning industry. Dihydroxyacetone is the active ingredient in commercial tanning agents that produce sunless tans. Dihydroxyacetone reacts with proteins in the skin, producing a complex colored pigment that gives the skin a brown hue. In addition, many commercial sunscreens are ketones. • Examples include avobenzone, oxybenzone, and dioxybenzone. Preparation of Aldehydes and Ketones Common Methods to Synthesize Aldehydes • Aldehydes are prepared from 1° alcohols, esters, acid chlorides, and alkynes. • By oxidation of 1° alcohols • By reduction of esters and acid chlorides • By hydroboration-oxidation of an alkyne Preparation of Aldehydes and Ketones Common Methods to Synthesize Ketones • Ketones are prepared from 2° alcohols, acid chlorides, and alkynes. • By oxidation of 2° alcohols with Cr6+ reagents. • By reaction of acid chlorides with organocuprates. • By Friedel–Crafts acylation. • By hydration of an alkyne. Aldehydes and ketones are also both obtained as products of the oxidative cleavage of alkenes. Reactions of Aldehydes and Ketones Reaction at the carbonyl carbon • Electrophilic carbonyl carbon makes aldehydes and ketones susceptible to nucleophilic addition reactions. • The elements of H and Nu are added to the carbonyl group. Reaction at the α-carbon • A second general reaction of aldehydes and ketones involves reaction at the α-carbon. A C—H bond on the α carbon to a carbonyl group is more acidic than many other C—H bonds, because reaction with base forms a resonance-stabilized enolate anion. Addition of 1° Amines Treatment of an aldehyde or ketone with a 1° amine affords an imine (also called a Schiff base). Nucleophilic attack of the 1° amine on the carbonyl group forms an unstable carbinolamine, which loses water to form an imine. The overall reaction results in replacement of C—O by C—NR. Addition of 1° Amines Many imines play vital roles in biological systems. A key molecule in the chemistry of vision is the highly conjugated imine rhodopsin, which is synthesized in the rod cells of the eye from 11-cis-retinal and a 1° amine in the protein opsin. Addition of 2° Amines A 2° amine reacts with an aldehyde or ketone to give an enamine. Enamines have a nitrogen atom bonded to a double bond (alkene + amine = enamine). Enamines are versatile intermediates. Because imines and enamines are formed by a set of reversible reactions, both can be converted back to carbonyl compounds by hydrolysis with mild acid. Addition of H2O—Hydration Treatment of a carbonyl compound with H2O in the presence of an acid or base catalyst adds the elements of H and OH across the carbon–oxygen π bond, forming a gem-diol (geminal-diols) or hydrate. • Hydration of a carbonyl group gives a good yield of gem-diol only with an unhindered aldehyde like formaldehyde, and with aldehydes containing nearby electron-withdrawing groups. • Geminal diols are a subclass of the diols, which in turn are a special class of alcohols. • The two hydroxyls in a geminal-diol are easily converted to a carbonyl or keto group C=O by loss of one water molecule, thus turning the diol into a ketone. Addition of Alcohols—Acetal Formation Aldehydes and ketones react with two equivalents of alcohol to form acetals. In an acetal, the carbonyl carbon from the aldehyde or ketone is now singly bonded to two OR" (alkoxy) groups. • The term acetal refers to any compound derived from an aldehyde or ketone, having two OR groups bonded to a single carbon Addition of Alcohols—Acetal Formation Conversion of an aldehyde or ketone to an acetal is a reversible reaction, so an acetal can be hydrolyzed to an aldehyde or ketone by treatment with aqueous acid. Because this reaction is also an equilibrium process, it is driven to the right by using a large excess of water for hydrolysis. Acetals as Protecting Groups Acetals are valuable protecting groups for aldehydes and ketones. • Acetals are easy to add and easy to remove, and they are stable to a wide variety of reaction conditions. • Acetals do not react with base, oxidizing agents, reducing agents, or nucleophiles. • Good protecting groups must survive a variety of reaction conditions that take place at other sites in a molecule, but they must also be selectively removed under mild conditions when needed. Cyclic Hemiacetals Although acyclic hemiacetals are generally unstable and therefore not present in appreciable amounts at equilibrium, cyclic hemiacetals containing five- and sixmembered rings are stable compounds that are readily isolated. Oxidation of Aldehydes Since aldehydes contain a hydrogen atom bonded directly to the carbonyl carbon, they can be oxidized to carboxylic acids; that is, the aldehyde C—H bond can be converted to a C—OH bond. Since ketones have no hydrogen atom bonded to the carbonyl group, they are not oxidized under similar reaction conditions. Carboxylic Acids and Esters Introduction, Nomenclature, Physical Properties, Interesting Carboxylic Acids, The Acidity of Carboxylic Acids (Reaction with Bases), Reactions Involving Carboxylic Acids and Esters (Ester Formation, Ester Hydrolysis) Introduction Carboxylic acids and esters are two families of organic molecules that contain a carbonyl group (C=O) singly bonded to an oxygen atom. • Carboxylic acid contains a carboxyl group (COOH). • An ester contains an alkoxy group (OR) bonded to the carbonyl carbon. Nomenclature of Carboxylic Acids and Esters To name carboxylic acids and esters, we must learn the suffix that identifies each functional group in the IUPAC system. Naming a Carboxylic Acid—RCOOH • In the IUPAC system, carboxylic acids are identified by the suffix - oic acid. • To name a carboxylic acid using the IUPAC system: 1. Find the longest chain containing the COOH group, and change the -e ending of the parent alkane to the suffix -oic acid. 2. Number the carbon chain to put the COOH group at C1, but omit this number from the name. Apply all of the other usual rules of nomenclature. Nomenclature of Carboxylic Acids and Esters Many simple carboxylic acids are often referred to by their common names. A common name uses the suffix -ic acid. Three common names are virtually always used. Nomenclature of Carboxylic Acids and Esters Naming a Ester—RCOOR‘ • The names of esters are derived from the names of the parent carboxylic acids. • Keep in mind that the common names formic acid, acetic acid, and benzoic acid are used for the parent acid, so these common parent names are used for their derivatives as well. • An ester has two parts to its structure, each of which must be named separately: • The RCO– group that contains the carbonyl group and is derived from a carboxylic acid, and an alkyl group (designated as R') bonded to the oxygen atom. Nomenclature of Carboxylic Acids and Esters Naming a Ester—RCOOR‘ • In the IUPAC system, esters are identified by the suffix -ate. • To name an ester: • Name the R' group bonded to the oxygen atom as an alkyl group. • Name the RCO– group by changing the -ic acid ending of the parent carboxylic acid to the suffix -ate. Physical Properties of Carboxylic Acids and Esters Carboxylic acids and esters are polar compounds because they possess a polar carbonyl group. Carboxylic acids also exhibit intermolecular hydrogen bonding since they possess a hydrogen atom bonded to an electronegative oxygen atom. Carboxylic acids often exist as dimers, held together by two intermolecular hydrogen bonds. Physical Properties of Carboxylic Acids and Esters Carboxylic acids have stronger intermolecular forces than esters, giving them higher boiling points and melting points, when comparing compounds of similar size. Carboxylic acids have higher boiling points and melting points than alcohols of comparable size. Physical Properties of Carboxylic Acids and Esters Like other oxygen-containing compounds, carboxylic acids and esters having fewer than six carbons are soluble in water. Higher molecular weight compounds are insoluble in water because the nonpolar portion of the molecule, the C—C and C—H bonds, gets larger than the polar carbonyl group. Interesting Carboxylic Acids Simple carboxylic acids have biting or foul odors. Formic acid (HCO2H) is responsible for the sting of some types of ants. Acetic acid (CH3CO2H) is the sour-tasting component of vinegar. Air oxidation of ethanol (CH3CH2OH) to acetic acid makes “bad” wine taste sour. Interesting Carboxylic Acids Several skin care products that purportedly smooth fine lines and improve skin texture contain α-hydroxy acids (alpha-hydroxy acids). • 𝛂-Hydroxy acids contain a hydroxyl group on the carbon bonded to the carboxyl group. • Two common α-hydroxy acids are glycolic acid and lactic acid. • Glycolic acid occurs naturally in sugarcane, and lactic acid gives sour milk its distinctive taste. Interesting Carboxylic Acids Aspirin and ibuprofen are common pain relievers and anti-inflammatory agents that contain a carboxyl group. • It was shown that aspirin blocks the synthesis of prostaglandins, carboxylic acids containing 20 carbons that are responsible for mediating pain, inflammation, and a wide variety of other biological functions. • Aspirin relieves pain and decreases inflammation because it prevents the synthesis of prostaglandins, the compounds responsible for both of these physiological responses, from arachidonic acid. The Acidity of Carboxylic Acids Carboxylic acids are acids; that is, they are proton donors. • When a carboxylic acid is dissolved in water, an acid–base reaction occurs: the carboxylic acid donates a proton to H2O, forming its conjugate base, a carboxylate anion, and water gains a proton, forming its conjugate acid, H3O+. • While carboxylic acids are more acidic than other families of organic compounds, they are weak acids compared to inorganic acids like HCl or H2SO4. Thus, only a small percentage of a carboxylic acid is ionized in aqueous solution. The Acidity of Carboxylic Acids Reaction with Bases • Carboxylic acids react with bases such as NaOH to form water-soluble salts. For example; The Acidity of Carboxylic Acids • The salts of carboxylic acids that are formed by acid–base reactions are water-soluble ionic solids. • Thus, a water-insoluble carboxylic acid like octanoic acid can be converted to its water soluble sodium salt by reaction with NaOH. The Acidity of Carboxylic Acids Salts of carboxylic acids are commonly used as preservatives. • Sodium benzoate, which inhibits the growth of fungus, is a preservative used in soft drinks. • Potassium sorbate is an additive that prolongs the shelf-life of baked goods and other foods. These salts do not kill bacteria or fungus. They increase the pH of the product, thus preventing further growth of microorganisms. Reactions Involving Carboxylic Acids and Esters Carboxylic acids and esters undergo a common type of reaction—substitution. When a carboxylic acid or ester (RCOZ) undergoes substitution, the group Z (Z = OH or OR') bonded to the carbonyl carbon is replaced by another group of atoms (Y=OR' or OH). Reactions Involving Carboxylic Acids and Esters Ester Formation • Treatment of a carboxylic acid (RCOOH) with an alcohol (R'OH) in the presence of an acid catalyst forms an ester (RCOOR'). This reaction is called a Fischer esterification. • Esterification is a substitution because the OR' group of an alcohol replaces the OH group of the starting carboxylic acid. Reactions Involving Carboxylic Acids and Esters Ethyl acetate, • It is the ester of ethanol and acetic acid. • It is a colorless liquid. • It has a characteristic sweet smell (similar to pear drops) • It is used in glues, nail polish removers, decaffeinating tea and coffee, and cigarettes (see list of additives in cigarettes). • It is manufactured on a large scale for use as a solvent. Reactions Involving Carboxylic Acids and Esters Ester Hydrolysis • Esters are hydrolyzed with water in the presence of acid or base. • Treatment of an ester (RCOOR') with water in the presence of an acid catalyst forms a carboxylic acid (RCOOH) and a molecule of alcohol (R'OH). • This reaction is a hydrolysis, since bonds are cleaved by reaction with water. The most prevalent naturally occurring esters are the triacylglycerols. • • Triacylglycerols contain three ester groups, each having a long carbon chain (abbreviated as R, R', and R") bonded to the carbonyl group. Triacylglycerols are lipids; that is, they are water-insoluble organic compounds found in biological systems. Animal fats and vegetable oils are composed of triacylglycerols. Amides Naming an Amide, Physical Properties, Amide hydrolysis, Interesting Amines and Amids, Epinephrine and related compounds, Penicillin Since it is associated with amines, it has been given with amines in the context of "Compounds Containing One Heteroatom Single Bond". In this note, only the Amid section is included. Amides Amides contain a carbonyl group bonded to a nitrogen atom. The N atom of an amide may be bonded to other hydrogen atoms or alkyl groups. Amides are classified as 1°, 2°, or 3° depending on the number of carbon atoms bonded directly to the nitrogen atom. A primary (1°) amide contains one C—N bond. A 1° amide has the structure RCONH2. A secondary (2°) amide contains two C—N bonds. A 2° amide has the structure RCONHR'. A tertiary (3°) amide contains three C—N bonds. A 3° amide has the structure RCONR'2. Naming an Amide In the IUPAC system, amides are identified by the suffix -amide. Naming an Amide A 2° or 3° amide has two parts to its structure: the RCO– group that contains the carbonyl and one or two alkyl groups bonded to the nitrogen atom. To name a 2° or 3° amide, • Name the alkyl group (or groups) bonded to the N atom of the amide. Use the prefix “N-” preceding the name of each alkyl group. • Name the RCO– group with the suffix -amide. Name the following amide: HCONHCH2CH3. Answer: N-ethylformamide Physical Properties 1° and 2° amides have higher boiling points and melting points than esters and 3° amides of comparable size. Amides having fewer than six carbons are soluble in water. Higher molecular weight amides are insoluble in water because the nonpolar portion of the molecule, the C—C and C—H bonds, gets larger than the polar carbonyl group. Amide Hydrolysis Treatment of an amide (RCONHR') with water in the presence of an acid catalyst (HCl) forms a carboxylic acid (RCOOH) and an ammonium salt (R'NH3+Cl–). Amide Hydrolysis Amides are also hydrolyzed in aqueous base to form carboxylate anions and a molecule of ammonia (NH3) or amine. Interesting Amines and Amides Caffeine and nicotine are widely used stimulants of the central nervous system that contain nitrogen atoms in rings. Caffeine and nicotine, like the amines, are alkaloids, naturally occurring amines derived from plant sources. Epinephrine and Related Compounds Epinephrine, or adrenaline as it is commonly called, is an amine synthesized in the adrenal glands from norepinephrine (noradrenaline). Epinephrine and Related Compounds When an individual senses danger or is confronted by stress, the hypothalamus region of the brain signals the adrenal glands to synthesize and release epinephrine, which enters the bloodstream and then stimulates a response in many organs. • Epinephrine synthesis occurs in the interior of the adrenal gland. Epinephrine release causes; increase in heart rate, increase in blood pressure, increase in glucose synthesis, dilation of lung passages. Epinephrine and Related Compounds The search for drugs that were structurally related to epinephrine but exhibited only some components of its wide range of biological activities led to the discovery of some useful medications. Both albuterol and salmeterol dilate lung passages; that is, they are bronchodilators. They do not, however, stimulate the heart. This makes both compounds useful for the treatment of asthma. Penicillin The antibiotic properties of penicillin were first discovered in 1928 by Sir Alexander Fleming, who noticed that a mold of the genus Penicillium inhibited the growth of certain bacteria. The penicillins are a group of related antibiotics. All penicillins contain two amide units. One amide is part of a four-membered ring called a 𝛃-lactam. The second amide is bonded to the four-membered ring. Penicillin The first penicillin to be discovered was penicillin G. Amoxicillin is another example in common use today. Penicillin interferes with the synthesis of the bacterial cell wall. References Eren, M. 2015. Organic Chemistry Lecture Papers (in TR) • Thanks to Prof. Dr. Meryem EREN’e teşekkürlerimle... Serpek, B. 2015. Organic Chemistry. Nobel Akademik Yayıncılık (TR) Smith JG (2010). Organic Chemistry, 3rd Edition, McGraw-Hill. Smith JG (2012). General, Organic, & Biological Chemistry 2nd Edition, McGraw-Hill.