* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Chemistry Chapter 1
Survey
Document related concepts
Transcript
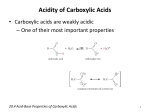
Organic Chemistry Chapter 10 Part I Carboxylic Acids and Their Derivatives (Ester) Nanoplasmonic Research Group Carboxylic Acids (General) carboxyl group three ways to represent a carboxylic acid acid derivatives • Organic Acids characterized the presence of a carboxylic group • Bronsted-Lowry acids: Proton donors • Salts or Anions: Carboxylates Nomenclature • In IUPAC nomenclature: carboxylic acids have an -oic acid suffix • In common nomenclature, the suffix is usually -ic acid Physical Properties of Carboxylic Acids • Polar and form hydrogen bonds with each other • At high T, in vapor phase, carboxylic acids usually exist as dimeric pairs • Water solubility: Chain-length dependent Acidity of Carboxylic Acids • The acidity can be explained by either the stability of the acids or the stability of the conjugate base using inductive effect or resonance effect • The stability of the acids: Inductive effect • The stability of the conjugate base: Both Synthesis of Carboxylic Acids • Oxidation of Primary Alcohols and Aldehydes • Hydrolysis of Nitriles, Esters, or Amides with The Addition of Acid or Base Roles of Acid or Base !!!!!! (page 298, 10.15) • Reaction of Grignard Reagents with Carbon Dioxide Carboxylic Acids Derivatives (Ester) • The R part of the -OR groups is named first , followed by the name of the acid, with the -ic ending changed to -ate • Ocurrence: the flavor and fragrance of many fruits and flowers • Hydrogen bond acceptor: Volatile than carboxylic acids • Cyclic ester: Lactone How to Prepare Ester ?? - Fischer Esterification - • Refluxing a carboxylic acid in an alcohol, which acts as both solvent and reactant • Since the reaction is an equilibrium, how to push the equilibrium to the right ???? le Chatelier’s principle !!! How to Push the equilibrium to the right ? - Fischer Esterification - • Using the alcohol as a solvent (in large excess) • Using Sulfuric Acid: It acts as an acid catalyst and as a dehydrating agent • Distillation (boiling point) What happens to Fischer Esterification ? • • • • Nucleophilic Acyl Substitution !!! Please take a look at eq. 10.19 on page 301 Protecting group Tetrahedral intermediate Reactions of Esters • Saponification, Reduction, Ammonolysis, and Reaction with Grignard Reagent