* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Isomerism of coordination compounds
Jahn–Teller effect wikipedia , lookup
Metal carbonyl wikipedia , lookup
Hydroformylation wikipedia , lookup
Spin crossover wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Cluster chemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
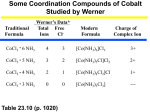
Isomerism of coordination compounds Structural isomerism Ionization isomerism When ligands are exchanged in a complex by the counter-ion, it is called ionization isomerism. e.g. [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl Linkage isomerism A ligand connects with the central metal atom through different atoms. e.g. the (NO2)- group can bond either through the nitrogen or through one of the oxygen atoms, so that a formula like [Co(NO2)(NH3)5]2+ might correspond to two molecules (here: nitro or nitrito complex). Coordination isomerism The metal ions in a compound with two complex ions exchange their places. e.g. [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6] Hydrate isomerism When water molecules inside or outside the complex change their position with a ligand, it is called hydrate isomerism. e.g. CrCl3•6H2O: [Cr(H2O)6]Cl3, [Cr(H2O)5Cl]Cl2•H2O and [Cr(H2O)4Cl2]Cl•2H2O Stereoisomerism Stereoisomers have the same molecular formula and connective sequence of the atoms, but they differ in spatial arrangements of their atoms. The most typical character of them is that they are not ‘superimposable’. Enantiomers: a pair of stereoisomers that are related to each other by reflection. Pure enantiomers are optically active: the plane of polarized light rotates in different directions when the light goes through them. They include no mirror-plane or inversion center. Diastereomers: stereoisomers which are not enantiomers. a) cis-trans: When a pair of identical ligands are arranged in opposing directions, the isomer is referred as trans, on the contrary, the isomer is referred as cis,. (octahedra or square planar complexes) b) fac-mer: When three identical ligands occupy the vertices of one face of an octahedron, the isomer is referred as fac(ial). If these three ligands together with the metal ion form a plane in the octahedron, the isomer is referred as mer(idional). Literature D.F. Shriver, P.W. Atkins, C.H. Langford, Inorganic Chemistry, Oxford University Press, 1994 * Bo Xin, Summary to the Seminar of the Lecture Advanced Inorganic Chemistry, Siegen 08/09