* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 22-Newest-CD
Molecular orbital wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Electron configuration wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Bond valence method wikipedia , lookup
Atomic theory wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
History of molecular theory wikipedia , lookup
Bent's rule wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Spin crossover wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Metallic bonding wikipedia , lookup
Chemical bond wikipedia , lookup
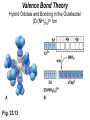
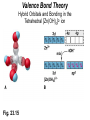
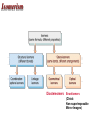
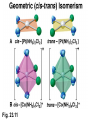
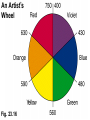
Some Coordination Compounds of Cobalt Studied by Werner Werner’s Data* Total Free Ions Cl- Traditional Formula . CoCl . 5 NH CoCl . 4 NH CoCl . 3 NH CoCl3 6 NH3 Modern Formula Charge of Complex Ion 4 3 [Co(NH3)6]Cl3 3+ 3 3 3 2 [Co(NH3)5Cl]Cl2 2+ 3 3 2 1 [Co(NH3)4Cl2]Cl 1+ 3 3 0 0 [Co(NH3)3Cl3] --- Table 23.10 (p. 1020) Valence Bond Theory Hybrid Orbitals and Bonding in the Octahedral [Cr(NH3)6]3+ Ion Fig. 23.13 Valence Bond Theory Hybrid Orbitals and Bonding in the Square Planar [Ni(CN)4]2- Ion Fig. 23.14 Valence Bond Theory Hybrid Orbitals and Bonding in the Tetrahedral [Zn(OH)4]2- ion Fig. 23.15 Isomerism QuickTime™ and a Sorenson Video decompressor are needed to see this picture. Isomerism • Isomers: two compounds with the same formulas but different arrangements of atoms. • Coordination-sphere isomers and linkage isomers: have different structures (i.e. different bonds). • Geometrical isomers and optical isomers are stereoisomers (i.e. have the same bonds, but different spatial arrangements of atoms). • Structural isomers have different connectivity of atoms. • Stereoisomers have the same connectivity but different spatial arrangements of atoms. Isomerism Diastereomers Enantiomers (Chiral: Non-superimposable Mirror Images) Isomerism Structural Isomerism Fig. 23.11 Stereoisomerism Fig. 23.12 Stereoisomerism • Enantiomers are chiral: I.e. They are non-superimposable mirror images. • Enantiomers are “optical isomers.” eg. (+) and (-) carvone • Most physical and chemical properties of enantiomers are identical. • Therefore, enantiomers are very difficult to separate eg. Tartaric acid…ask Louis Pasteur. • Enzymes are catalysts that are very specific, acting on only one enantiomer. • Enantiomers can have very different physiological effects: eg. (+) and (-) carvone Chirality QuickTime™ and a Sorenson Video decompressor are needed to see this picture. Optical Activity QuickTime™ and a Sorenson Video decompressor are needed to see this picture. Optical Activity (+) dextrorotatory (-) levorotatory The mirror image of an enantiomer will rotate the plane of polarized light by the same amount in the opposite direction. Eg (+) d-carvone +62o and (-) l-carvone -62o…. What about a 50:50 (racemic) mixture? Color and Magnetism • Color of a complex depends on: (i) the metal and (ii) its oxidation state. • Pale blue [Cu(H2O)6]2+ can be converted into dark blue [Cu(NH3)6]2+ by adding NH3(aq). • A partially filled d orbital is usually required for a complex to be colored. • So, d 0 metal ions are usually colorless. Exceptions: MnO4- and CrO42-. • Colored compounds absorb visible light. • The color our eye perceives is the sum of the light not absorbed by the complex. Color and Magnetism Color An Artist’s Wheel Fig. 23.16 Relation Between Absorbed and Observed Colors Absorbed Color (nm) Violet Blue Blue-green Yellow-green Yellow Orange Red 400 450 490 570 580 600 650 Table 23.11 (p. 1027) Observed Color (nm) Green-yellow Yellow Red Violet Dark blue Blue Green 560 600 620 410 430 450 520 Color & Visible Spectra Color& Spectra • The plot of absorbance versus wavelength is the absorption spectrum. • For example, the absorption spectrum for [Ti(H2O)6]3+ has a maximum absorption occurs at 510 nm (green and yellow). • So, the complex transmits all light except green and yellow. • Therefore, the complex appears to be purple. Color and Magnetism Magnetism • Many transition metal complexes are paramagnetic (i.e. they have unpaired electrons). • There are some interesting observations. Consider a d6 metal ion: – [Co(NH3)6]3+ has no unpaired electrons, but [CoF6]3has four unpaired electrons per ion. • We need to develop a bonding theory to account for both color and magnetism in transition metal complexes. Crystal-Field Theory QuickTime™ and a Intel Indeo® Video R3.2 decompressor are needed to see this picture.