Basic physics of high harmonic generation (HHG)
... The emission from the atom/molecule is determined by the dipole driven at the harmonic frequency ψ (t) = ψ0(t) + ψc(t) Time dependent wavefunction is a superpositon of continuum and bound parts. Contains components oscillating at high frequencies – leading to emission at these frequencies Single at ...
... The emission from the atom/molecule is determined by the dipole driven at the harmonic frequency ψ (t) = ψ0(t) + ψc(t) Time dependent wavefunction is a superpositon of continuum and bound parts. Contains components oscillating at high frequencies – leading to emission at these frequencies Single at ...
Worksheet Key - UCSB C.L.A.S.
... 11. An electron is excited from the ground state to the n = 3 state in a hydrogen atom. Which of the following statement(s) is/are true? a. It takes more energy to ionize the electron from n= 3 than from the ground state. b. The electron is farther from the nucleus on average in the n = 3 state than ...
... 11. An electron is excited from the ground state to the n = 3 state in a hydrogen atom. Which of the following statement(s) is/are true? a. It takes more energy to ionize the electron from n= 3 than from the ground state. b. The electron is farther from the nucleus on average in the n = 3 state than ...
Chemistry 1000 Lecture 6: Quantum mechanics and spectroscopy
... Prediction: particles (electrons, neutrons, etc.) should diffract like light under appropriate conditions Modern methods based on this fact: transmission electron ...
... Prediction: particles (electrons, neutrons, etc.) should diffract like light under appropriate conditions Modern methods based on this fact: transmission electron ...
7.4 The Wave Nature of Matter * 7.5 Quantum Mechanics and the Atom
... • Since we can not determine the exact location and velocity of an electron at the same time, experimentation has been done over time to identify the most likely places that the electrons exist in an atom. These locations are called orbitals. • Schrödinger's equation can be used derive the energies ...
... • Since we can not determine the exact location and velocity of an electron at the same time, experimentation has been done over time to identify the most likely places that the electrons exist in an atom. These locations are called orbitals. • Schrödinger's equation can be used derive the energies ...
Chemistry 1 Practice Final Exam - Tutor
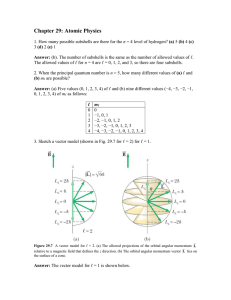
... n = 2, l = 1, ml = 1 : n = 4, l = 2, ml = -2 : n = 3, l = 1, ml = 2 : n = 2, l = 2, ml = -1 ...
... n = 2, l = 1, ml = 1 : n = 4, l = 2, ml = -2 : n = 3, l = 1, ml = 2 : n = 2, l = 2, ml = -1 ...
chemistry-study-guide-grade
... Explain how a bond forms in terms of atomic orbitals. Use the Lewis structure and number of electron domains of a compound to determine the hybridization of its bonds. 9. Provide the number of sigma and pi bonds in a molecule or around a central atom. 10. Give in your own words the major relevance o ...
... Explain how a bond forms in terms of atomic orbitals. Use the Lewis structure and number of electron domains of a compound to determine the hybridization of its bonds. 9. Provide the number of sigma and pi bonds in a molecule or around a central atom. 10. Give in your own words the major relevance o ...
PHYSICS 215 - Thermodynamics and Modern Physics Name:
... Speed of light, c = 3.00E8 m/s Charge of an electron, -e = -1.6E-19 C Mass of the electron, me = 9.1E-31 kg = 511 keV/c2 = 5.49E-4 u Mass of the proton, mp = 1.67E-27 kg = 938 MeV/c2 = 1.00728 u Mass of the α particle, mα = 3727.4 MeV/c2 = 4.00151 u ...
... Speed of light, c = 3.00E8 m/s Charge of an electron, -e = -1.6E-19 C Mass of the electron, me = 9.1E-31 kg = 511 keV/c2 = 5.49E-4 u Mass of the proton, mp = 1.67E-27 kg = 938 MeV/c2 = 1.00728 u Mass of the α particle, mα = 3727.4 MeV/c2 = 4.00151 u ...
Midterm Exam 2
... easily, leading to even greater reactivity. In contrast diatomic nitrogen, N2, is almost completely unreactive under standard condition in a manner similar to the noble gases. Due to this N2 is used most often as a preservation or preventing agent, with its primary function to prevent reactive speci ...
... easily, leading to even greater reactivity. In contrast diatomic nitrogen, N2, is almost completely unreactive under standard condition in a manner similar to the noble gases. Due to this N2 is used most often as a preservation or preventing agent, with its primary function to prevent reactive speci ...
The Quantum Atom (section 18)
... Section 18: The Quantum Atom JJ Thomson 1899 – discovered electron using cathode ray tube (diagrams p210 Adams& Allday, p784 Muncaster). Particles given off from cathode always have the same charge to mass ratio, no matter what element the cathode is made of. Conclusion – they are subatomic particle ...
... Section 18: The Quantum Atom JJ Thomson 1899 – discovered electron using cathode ray tube (diagrams p210 Adams& Allday, p784 Muncaster). Particles given off from cathode always have the same charge to mass ratio, no matter what element the cathode is made of. Conclusion – they are subatomic particle ...
Unit 1 Inorganic Flashcards
... concentration of a particular metal element in a sample. The electrons are promoted to higher energy levels by absorbing energy, and the wavelength of the absorbed energy can be used to determine which element is present. The intensity of the absorbed light can be used to determine the concentration ...
... concentration of a particular metal element in a sample. The electrons are promoted to higher energy levels by absorbing energy, and the wavelength of the absorbed energy can be used to determine which element is present. The intensity of the absorbed light can be used to determine the concentration ...
3.8 Case study: 21 cm line in the interstellar medium
... where NA,Z,n is the number density of atoms of the element A in the ionization state Z. Taking into account that the most abundant heavy elements (C, O, Si etc.) are fully ionized at T & 106 K, one can assume that the absorption is mostly determined by the hydrogen-like ions of heavy elements. Accor ...
... where NA,Z,n is the number density of atoms of the element A in the ionization state Z. Taking into account that the most abundant heavy elements (C, O, Si etc.) are fully ionized at T & 106 K, one can assume that the absorption is mostly determined by the hydrogen-like ions of heavy elements. Accor ...
Physics IV - Exam - Winter 2007/08 Please note:
... 6. Sunlight arrives at the earth at 1.4 kWm−2 when the sun is directly overhead. The distance between the earth and the sun is 1.5 × 1011 m, and the sun’s radius is 7 × 108 m. (a) Assuming that the sun is a perfect black body, show that it’s surface temperature is TS = 5800 K. Sketch the spectrum o ...
... 6. Sunlight arrives at the earth at 1.4 kWm−2 when the sun is directly overhead. The distance between the earth and the sun is 1.5 × 1011 m, and the sun’s radius is 7 × 108 m. (a) Assuming that the sun is a perfect black body, show that it’s surface temperature is TS = 5800 K. Sketch the spectrum o ...
(s) If 5.00 moles of zinc is placed into 1.50 L... 34. solution,what is the mass of the hydrogen gas produced?
... Base your answers to questions 34 through 32 on on the following chemical reaction: ...
... Base your answers to questions 34 through 32 on on the following chemical reaction: ...
Atomic Radii Answers File
... charge has not changed. However, now the nucleus is attracting one less electron so the remaining ones are pulled in closer. When an atom gains an electron to form a negative ion, the nuclear charge has not changed. However, now the nucleus is attracting one more electron so they are not pulled as s ...
... charge has not changed. However, now the nucleus is attracting one less electron so the remaining ones are pulled in closer. When an atom gains an electron to form a negative ion, the nuclear charge has not changed. However, now the nucleus is attracting one more electron so they are not pulled as s ...
direct electron acceleration by chirped laser pulse in the regime of
... [email protected] Now technology of chirped-pulse amplification makes it possible to achieve multi-TW and even PW-class laser system. Super power laser pulses are used for high energy electron and ion generation. Fluxes of accelerated particles are demanded for various applications including medici ...
... [email protected] Now technology of chirped-pulse amplification makes it possible to achieve multi-TW and even PW-class laser system. Super power laser pulses are used for high energy electron and ion generation. Fluxes of accelerated particles are demanded for various applications including medici ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.