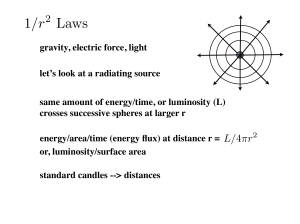
AP Unit 1 Test Review
... 3. The ground-state configuration for the atoms of a transition element 4. The ground-state configuration of a negative ion of a halogen 5. The ground-state configuration of a common ion of an alkaline earth element 6. Which of the following sets of quantum numbers (n, l, ml, ms) best describes the ...
... 3. The ground-state configuration for the atoms of a transition element 4. The ground-state configuration of a negative ion of a halogen 5. The ground-state configuration of a common ion of an alkaline earth element 6. Which of the following sets of quantum numbers (n, l, ml, ms) best describes the ...
Chapter 5 Worksheet 1
... The positions of very small objects like electrons can only be studied accurately by hitting them with very high energy (x-rays, gamma rays, etc.) and since the electron is so small it will have its momentum (motion) changed to a large extent by this high energy wave. 3. What is wave particle dualit ...
... The positions of very small objects like electrons can only be studied accurately by hitting them with very high energy (x-rays, gamma rays, etc.) and since the electron is so small it will have its momentum (motion) changed to a large extent by this high energy wave. 3. What is wave particle dualit ...
Electron Configuration
... The present-day model of the atom, in which electrons are located in orbitals, is also known as the quantum model ◦ States electrons within an energy level are located in orbitals, regions of high probability for finding a particular electrons. ◦ Does not, however, explain how the electrons move abo ...
... The present-day model of the atom, in which electrons are located in orbitals, is also known as the quantum model ◦ States electrons within an energy level are located in orbitals, regions of high probability for finding a particular electrons. ◦ Does not, however, explain how the electrons move abo ...
homework 2, due October 3rd
... Consider a particle described at some particular instant of time by the wave function ψ(x) = Ae−ax . 1. Determine A so ψ is normalized. 2. Compute hxi, hx2 i and σx2 = h(x − hxi)2 i. 3. Compute hpi, hp2 i and σp2 = h(p − hpi)2 i. 4. Show that by changing a one can make either σx2 or σp2 small, but n ...
... Consider a particle described at some particular instant of time by the wave function ψ(x) = Ae−ax . 1. Determine A so ψ is normalized. 2. Compute hxi, hx2 i and σx2 = h(x − hxi)2 i. 3. Compute hpi, hp2 i and σp2 = h(p − hpi)2 i. 4. Show that by changing a one can make either σx2 or σp2 small, but n ...
final2012
... orbital shell type and total angular momentum, rather than shell number. (Hint – all of the levels that are filled have n=1) b) Which is the unstable isotope and why. c) Write down the decay mechanism that converts the unstable to the stable isotope. d) Calculate the nuclear radius for these isotope ...
... orbital shell type and total angular momentum, rather than shell number. (Hint – all of the levels that are filled have n=1) b) Which is the unstable isotope and why. c) Write down the decay mechanism that converts the unstable to the stable isotope. d) Calculate the nuclear radius for these isotope ...
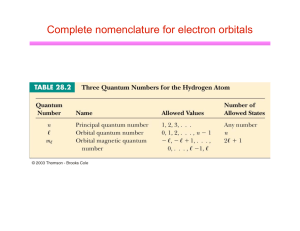
Complete nomenclature for electron orbitals
... hydrogen (or any other type of atom) can be specified by the quantum numbers: n,l,ml and ms l Wolfgang Pauli (one of founders of quantum mechanics) discovered that no two electrons in the same atom can ever have exactly the same values for the set of quantum numbers u u ...
... hydrogen (or any other type of atom) can be specified by the quantum numbers: n,l,ml and ms l Wolfgang Pauli (one of founders of quantum mechanics) discovered that no two electrons in the same atom can ever have exactly the same values for the set of quantum numbers u u ...
CHM 101 - Academic Computer Center
... high, positive or slightly negative low, positive or slightly negative high, very negative None of these is generally correct. ...
... high, positive or slightly negative low, positive or slightly negative high, very negative None of these is generally correct. ...
The Periodic Table - Mrs Molchany`s Webpage
... Reason: electrons added in the same principal quantum level do not completely shield the increasing nuclear charge caused by the added protons. The electrons in the same principal quantum level are generally more strongly bound when moving left to right across the periodic table ...
... Reason: electrons added in the same principal quantum level do not completely shield the increasing nuclear charge caused by the added protons. The electrons in the same principal quantum level are generally more strongly bound when moving left to right across the periodic table ...
Quantum Atom
... Quantum Mechanics Describes mathematically the properties of an electron Wave function (Ψ2) – series of solutions that describes the allowed energy levels for electrons Shows regions of probability of finding an electron Regions of high electron density have large values of Ψ2 ...
... Quantum Mechanics Describes mathematically the properties of an electron Wave function (Ψ2) – series of solutions that describes the allowed energy levels for electrons Shows regions of probability of finding an electron Regions of high electron density have large values of Ψ2 ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.