4-1 The lowest energy state of an atom is its ground state. (usually
... The lowest energy state of an atom is its ground state. (usually it’s the lowest levels) ...
... The lowest energy state of an atom is its ground state. (usually it’s the lowest levels) ...
Slow Photoelectron Imaging
... Rydberg electron with the core, which implies the image only weakly depends on the M of the initial state. Hence Xe behaves like an atom with a 1 S core in this experiment, and the images can be qualitatively reproduced by a model without multichannel couplings in zero field. The full quantum calcul ...
... Rydberg electron with the core, which implies the image only weakly depends on the M of the initial state. Hence Xe behaves like an atom with a 1 S core in this experiment, and the images can be qualitatively reproduced by a model without multichannel couplings in zero field. The full quantum calcul ...
Bohr Model of the Atom
... The Danish physicist Niels Bohr, who first presented this model of the atom, based it on 3 fundamental postulates. (1) Electrons move around the nucleus in circular non-radiating orbits - called “stationary states”. However, they are not at rest! (2) An atom only emits or absorbs electromagnetic rad ...
... The Danish physicist Niels Bohr, who first presented this model of the atom, based it on 3 fundamental postulates. (1) Electrons move around the nucleus in circular non-radiating orbits - called “stationary states”. However, they are not at rest! (2) An atom only emits or absorbs electromagnetic rad ...
ELECTRONIC STRUCTURE OF ATOMS
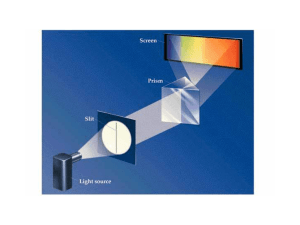
... When white light is separated, it forms a continuous spectrum Not all radiation is continuous. A spectrum where we see only specific wavelengths is called a line spectrum. Bohr used this observation to hypothesize that electrons were confined to specific energy states , orbits. ...
... When white light is separated, it forms a continuous spectrum Not all radiation is continuous. A spectrum where we see only specific wavelengths is called a line spectrum. Bohr used this observation to hypothesize that electrons were confined to specific energy states , orbits. ...
Which scientist developed the quantum mechanical model of the
... Which of the following states that no more than two electrons can occupy an atomic orbital and that two electrons in the same orbital must have opposite spins? A) B) C) D) ...
... Which of the following states that no more than two electrons can occupy an atomic orbital and that two electrons in the same orbital must have opposite spins? A) B) C) D) ...
4 slides per page() - Wayne State University Physics and
... The lines are dependent on the metal The lines are called characteristic xx-rays ...
... The lines are dependent on the metal The lines are called characteristic xx-rays ...
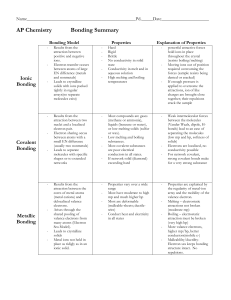
Types of Bonding Summary
... electrons. Arises through the shared pooling of valence electrons from many atoms (Electron Sea Model). Leads to crystalline solids Metal ions not held in place as ridigly as in an ionic solid. ...
... electrons. Arises through the shared pooling of valence electrons from many atoms (Electron Sea Model). Leads to crystalline solids Metal ions not held in place as ridigly as in an ionic solid. ...
No Slide Title
... Dp = momentum uncertainty (p = mv) h = Planck’s constant The uncertainty principle means that we can never simultaneously know the position (radius) and momentum (energy) of an electron, as defined in the Bohr model of the atom. ...
... Dp = momentum uncertainty (p = mv) h = Planck’s constant The uncertainty principle means that we can never simultaneously know the position (radius) and momentum (energy) of an electron, as defined in the Bohr model of the atom. ...
Atomic Structure Zumdahl Chemistry Chapter 7
... them. The significance of these results is that matter and energy are not distinct. Energy is really a form of matter, and all matter shows the same types of properties. That is all matter exhibited both particulate and wave properties. Large pieces of matter, exhibit predominantly particulate prope ...
... them. The significance of these results is that matter and energy are not distinct. Energy is really a form of matter, and all matter shows the same types of properties. That is all matter exhibited both particulate and wave properties. Large pieces of matter, exhibit predominantly particulate prope ...
Quantum Mechanical Model - Elmwood Park Memorial Middle School
... • Schrodinger created an equation for solving these locations, but it has only been completely solved for hydrogen Why hydrogen? The modern model of the atom is similar to Bohr s Model. The main difference is that there are no ORBITS! ...
... • Schrodinger created an equation for solving these locations, but it has only been completely solved for hydrogen Why hydrogen? The modern model of the atom is similar to Bohr s Model. The main difference is that there are no ORBITS! ...
Atomic Physics - SFSU Physics & Astronomy
... • Lowest energy state = “ground state” • Higher states = “excited states” • Photon energy equals difference in state energies • Hydrogen atom example – Energy levels – Line spectra ...
... • Lowest energy state = “ground state” • Higher states = “excited states” • Photon energy equals difference in state energies • Hydrogen atom example – Energy levels – Line spectra ...
Electronic Structure and the Periodic Table A. Bohr Model of the
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.