HW 10: Electron Configuration Practice -
... Think about the arrangement of electrons and which atom this configuration would represent. In quantum mechanics, the electron configuration is the arrangement of electrons around the nucleus of the atom. An electron configuration provides information about the number of electrons in each orbital. T ...
... Think about the arrangement of electrons and which atom this configuration would represent. In quantum mechanics, the electron configuration is the arrangement of electrons around the nucleus of the atom. An electron configuration provides information about the number of electrons in each orbital. T ...
energy levels
... energy levels in an atom – Tells you how far away the electron is from the nucleus – There are 1-7 energy levels, correlates with period numbers – Each level has same n number of sublevels – Maximum number of 2n2 electrons per level ...
... energy levels in an atom – Tells you how far away the electron is from the nucleus – There are 1-7 energy levels, correlates with period numbers – Each level has same n number of sublevels – Maximum number of 2n2 electrons per level ...
Matter Vocab Part 4
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
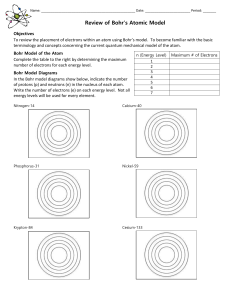
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. ...
... In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. ...
Quantum Cloud Model
... The original location of the electron is referred to as the Ground State The higher level is called the Excited State When the electron returns to the ground state it gives off energy equal to the difference between the two levels (excited and ground state) as electromagnetic radiation with its spec ...
... The original location of the electron is referred to as the Ground State The higher level is called the Excited State When the electron returns to the ground state it gives off energy equal to the difference between the two levels (excited and ground state) as electromagnetic radiation with its spec ...
Physics 12 Assignmen.. - hrsbstaff.ednet.ns.ca
... 2. In Rutherford’s planetary model of the atom, what keeps the electrons from flying off into space? In Rutherford’s planetary model of the atom, the Coulomb (or electrostatic) force keeps the electrons from flying off into space. Since the protons in the center are positively charged, the negativel ...
... 2. In Rutherford’s planetary model of the atom, what keeps the electrons from flying off into space? In Rutherford’s planetary model of the atom, the Coulomb (or electrostatic) force keeps the electrons from flying off into space. Since the protons in the center are positively charged, the negativel ...
Chapter 2 Part 1 ppt
... • 4r2R2: probability of finding electron at a given distance from nucleus, summed over all angles • Probability of finding the electron at a certain distance from the nucleus is not equal to the probability of finding the electron at a certain point at that distance from the nucleus. • There is a w ...
... • 4r2R2: probability of finding electron at a given distance from nucleus, summed over all angles • Probability of finding the electron at a certain distance from the nucleus is not equal to the probability of finding the electron at a certain point at that distance from the nucleus. • There is a w ...
Chapter 5
... Trends in atomic size (atoms in the same group, atoms in the same row); explanation for trends Definition of first ionization energy and higher ionization energies Trends in first ionization energy (atoms in the same group, atoms in the same row); explanation for trends Jumps in higher ionization en ...
... Trends in atomic size (atoms in the same group, atoms in the same row); explanation for trends Definition of first ionization energy and higher ionization energies Trends in first ionization energy (atoms in the same group, atoms in the same row); explanation for trends Jumps in higher ionization en ...
Orbital
... emission of light with wavelengths around 650 nm when strontium salts such as Sr(NO3)2 and SrCO3 are heated. (This can be easily demonstrated in the lab by dissolving ones of these salts in methanol that contains a little water and igniting the mixture in an evaporating dish.) Calculate the frequenc ...
... emission of light with wavelengths around 650 nm when strontium salts such as Sr(NO3)2 and SrCO3 are heated. (This can be easily demonstrated in the lab by dissolving ones of these salts in methanol that contains a little water and igniting the mixture in an evaporating dish.) Calculate the frequenc ...
Review for second exam:
... Trends in atomic size (atoms in the same group, atoms in the same row); explanation for trends Definition of first ionization energy and higher ionization energies Trends in first ionization energy (atoms in the same group, atoms in the same row); explanation for trends Jumps in higher ionization en ...
... Trends in atomic size (atoms in the same group, atoms in the same row); explanation for trends Definition of first ionization energy and higher ionization energies Trends in first ionization energy (atoms in the same group, atoms in the same row); explanation for trends Jumps in higher ionization en ...
Atomic Spectra
... one-electron situation such as He+, Li+2, Be+3) can be found from the equation: ...
... one-electron situation such as He+, Li+2, Be+3) can be found from the equation: ...
Modern Model of the Atom
... the position and momentum of an electron or any other particle with any great degree of certainty ...
... the position and momentum of an electron or any other particle with any great degree of certainty ...
Chem 1a Midterm Review
... Ions: all transition metals when they ionize the first two electrons that are lost are from the ns shell not the (n-1)d shell. Filling orbitals 1. Pauli Principle: Every electron must have a unique set of 4 quantum numbers 2. Aufbau principle: Fill lowest energy orbitals first 3. Hund's Rule: In a d ...
... Ions: all transition metals when they ionize the first two electrons that are lost are from the ns shell not the (n-1)d shell. Filling orbitals 1. Pauli Principle: Every electron must have a unique set of 4 quantum numbers 2. Aufbau principle: Fill lowest energy orbitals first 3. Hund's Rule: In a d ...
SEMESTER 1 EXAM Prblms/Short Ans
... Eq, S, U, SF – Equations; Show all equations used in all calculations. Steps; show each step leading to each answer. Units; show the appropriate units with each number used in all calculations. SF; Use the correct significant figures when expressing all answers. Use Scientific Notation for all numbe ...
... Eq, S, U, SF – Equations; Show all equations used in all calculations. Steps; show each step leading to each answer. Units; show the appropriate units with each number used in all calculations. SF; Use the correct significant figures when expressing all answers. Use Scientific Notation for all numbe ...
Photosynthesis Stores Energy in Organic Compounds
... I absorbs light energy while steps 1 & 2 are taking place Electron is excited, moves to a high-energy electron acceptor Excited electron is replaced by an electron from the end of the electron transport system ...
... I absorbs light energy while steps 1 & 2 are taking place Electron is excited, moves to a high-energy electron acceptor Excited electron is replaced by an electron from the end of the electron transport system ...
MSE 221 Quantum Physics of Materials
... MSE 221 Quantum Physics of Materials Elementary quantum concepts, wave mechanics, energy states, bonding, transitions, electronic properties Prerequisite: PHYS 151 14 weeks, lectures (3 times a week), recitation (once a week) Introduction Old Quantum Theory, Bohr model, De Broglie, duality Interfere ...
... MSE 221 Quantum Physics of Materials Elementary quantum concepts, wave mechanics, energy states, bonding, transitions, electronic properties Prerequisite: PHYS 151 14 weeks, lectures (3 times a week), recitation (once a week) Introduction Old Quantum Theory, Bohr model, De Broglie, duality Interfere ...
Baby-Quiz
... l – orbital quantum number, is related to the magnitude of the angular momentum of the electron; at given n can take integer values from 0 to (n-1); ml – magnetic quantum number, is related to the direction of the electron’s angular momentum, and it can take an integer values from –l to +l. ...
... l – orbital quantum number, is related to the magnitude of the angular momentum of the electron; at given n can take integer values from 0 to (n-1); ml – magnetic quantum number, is related to the direction of the electron’s angular momentum, and it can take an integer values from –l to +l. ...
Photosynthesis Stores Energy in Organic Compounds
... I absorbs light energy while steps 1 & 2 are taking place Electron is excited, moves to a high-energy electron acceptor Excited electron is replaced by an electron from the end of the electron transport system ...
... I absorbs light energy while steps 1 & 2 are taking place Electron is excited, moves to a high-energy electron acceptor Excited electron is replaced by an electron from the end of the electron transport system ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.