PHY4604–Introduction to Quantum Mechanics Fall 2004 Practice
... states which are degenerate in zero field by an amount so as to maximally absorb light of wavelength λ? e H = −µ · B = S · B m e e = = Sz Bz = h̄ms Bz , m m where the last step where the operator is replaced by its eigenvalues holds only when applied to Sz eigenstates, and where I have used ms for t ...
... states which are degenerate in zero field by an amount so as to maximally absorb light of wavelength λ? e H = −µ · B = S · B m e e = = Sz Bz = h̄ms Bz , m m where the last step where the operator is replaced by its eigenvalues holds only when applied to Sz eigenstates, and where I have used ms for t ...
Quantum Physics Cumulative Review
... 1. How was Einstein able to apply Planck’s idea that light waves had quantized energy to explain why some wavelengths of light could knock electrons off a block of a particular metal and create a photocurrent and others couldn’t? 2. How does the law of Conservation of Energy apply to a light beam hi ...
... 1. How was Einstein able to apply Planck’s idea that light waves had quantized energy to explain why some wavelengths of light could knock electrons off a block of a particular metal and create a photocurrent and others couldn’t? 2. How does the law of Conservation of Energy apply to a light beam hi ...
Chemistry Outcomes - hrsbstaff.ednet.ns.ca
... Explain the hydrogen line spectrum in terms of Bohr Model of the atom State two differences between the Bohr model and the quantum mechanical model of the atom Draw an energy level diagram for a given atom Define valence shell and valence electrons Label the sublevels on an energy level diagram with ...
... Explain the hydrogen line spectrum in terms of Bohr Model of the atom State two differences between the Bohr model and the quantum mechanical model of the atom Draw an energy level diagram for a given atom Define valence shell and valence electrons Label the sublevels on an energy level diagram with ...
Quantum Mechanical Model of the Atom
... And wherever charged particles are accelerated And atoms continuously emit photons due to their collisions with each other ...
... And wherever charged particles are accelerated And atoms continuously emit photons due to their collisions with each other ...
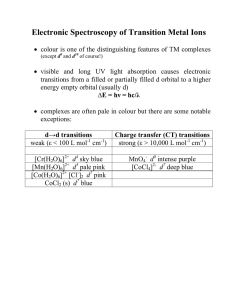
Electronic Spectroscopy of Transition Metal Ions
... in order to understand the spectroscopy of d ions with more than one d electron we must take the effect of e- - e- repulsion into account (we have ignored this so far) ...
... in order to understand the spectroscopy of d ions with more than one d electron we must take the effect of e- - e- repulsion into account (we have ignored this so far) ...
Glossary Chapter 4
... electromagnetic radiation a form of energy that exhibits wavelike behavior as it travels through space (91) electromagnetic spectrum all the forms of electromagnetic radiation (91) electron configuration the arrangement of electrons in an atom (105) excited state a state in which an atom has a highe ...
... electromagnetic radiation a form of energy that exhibits wavelike behavior as it travels through space (91) electromagnetic spectrum all the forms of electromagnetic radiation (91) electron configuration the arrangement of electrons in an atom (105) excited state a state in which an atom has a highe ...
Definitions are in Book
... energy to change their electron configurations. You’ll notice that it takes a lot of energy to remove an electron and even takes energy to add an electron. This stability is a direct result of the filling of the p-orbital. 2) How are Hess’s law, ∆Hof, and the fact that enthalpy is a state function a ...
... energy to change their electron configurations. You’ll notice that it takes a lot of energy to remove an electron and even takes energy to add an electron. This stability is a direct result of the filling of the p-orbital. 2) How are Hess’s law, ∆Hof, and the fact that enthalpy is a state function a ...
Electron Configurations
... • According to the Heisenberg Uncertainty Principle we can not know the exact position and motion of electrons with complete certainty. • We can only describe the probable locations of electrons. • We will describe the location of electrons when the atom is at its lowest energy . • These are called ...
... • According to the Heisenberg Uncertainty Principle we can not know the exact position and motion of electrons with complete certainty. • We can only describe the probable locations of electrons. • We will describe the location of electrons when the atom is at its lowest energy . • These are called ...
1 - shawnschmitt
... g. Mole- the amount of particles in 12g of Carbon-12, also, the amount of substance having 6.022x1023 of any kind of particle h. half-life- the amount of time required for ½ of the mass of an isotope to decay i. metalloid- those elements that have properties of both metals and nonmetals j. Ionizatio ...
... g. Mole- the amount of particles in 12g of Carbon-12, also, the amount of substance having 6.022x1023 of any kind of particle h. half-life- the amount of time required for ½ of the mass of an isotope to decay i. metalloid- those elements that have properties of both metals and nonmetals j. Ionizatio ...
File
... distinguishes one series of lines from another? (c) Draw an electronic energy level diagram for the hydrogen atom and indicate on it the transition corresponding to the line of lowest frequency in the Balmer series. (d) What is the difference between an emission spectrum and an absorption spectrum? ...
... distinguishes one series of lines from another? (c) Draw an electronic energy level diagram for the hydrogen atom and indicate on it the transition corresponding to the line of lowest frequency in the Balmer series. (d) What is the difference between an emission spectrum and an absorption spectrum? ...
What is matter made of?
... Said that electrons orbit the nucleus along certain paths called energy levels or orbitals. Chemical properties are determined by the electrons in the outermost orbit. ...
... Said that electrons orbit the nucleus along certain paths called energy levels or orbitals. Chemical properties are determined by the electrons in the outermost orbit. ...
4. - period2chem
... Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they emit photons which correspond to the lines in the emission spectrum. The more energy lost, the more energy the photon has. Bohr’s model stated that electrons ...
... Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they emit photons which correspond to the lines in the emission spectrum. The more energy lost, the more energy the photon has. Bohr’s model stated that electrons ...
Above-threshold ionization in a strong dc electric field
... 1016 W / cm2 have been realized at photon frequencies comparable to or larger than the atomic ionization potential, leading to dramatically different and as-yet not fully explained behavior 关6兴. In this paper we report photoelectron imaging experiments at far-infrared wavelengths 共 = 108 m, = 4. ...
... 1016 W / cm2 have been realized at photon frequencies comparable to or larger than the atomic ionization potential, leading to dramatically different and as-yet not fully explained behavior 关6兴. In this paper we report photoelectron imaging experiments at far-infrared wavelengths 共 = 108 m, = 4. ...
QUANTUM NUMBERS
... For an electron in an atom with l=0 is said to be in an s state. For an electron in an atom with l=1 is said to be in an p state. For an electron in an atom with l=2 is said to be in an d state. For an electron in an atom with l=3 is said to be in an e state. ...
... For an electron in an atom with l=0 is said to be in an s state. For an electron in an atom with l=1 is said to be in an p state. For an electron in an atom with l=2 is said to be in an d state. For an electron in an atom with l=3 is said to be in an e state. ...
5 ELECTRONS IN ATOMS Vocabulary Review Name ___________________________
... 3. the SI unit of frequency ...
... 3. the SI unit of frequency ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.