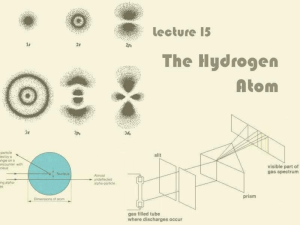
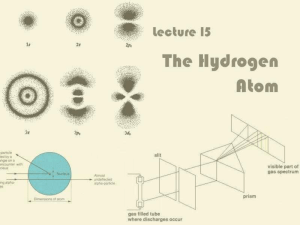
Lecture 15: The Hydrogen Atom
... Hydrogen Atom is Unstable? It is known that accelerating charges emit radiation Thus, electron should emit radiation, lose energy and eventually fall into the nucleus! Why doesn’t this happen? Shows that something was wrong with this model of the hydrogen atom ...
... Hydrogen Atom is Unstable? It is known that accelerating charges emit radiation Thus, electron should emit radiation, lose energy and eventually fall into the nucleus! Why doesn’t this happen? Shows that something was wrong with this model of the hydrogen atom ...
Matter and Energy Identify a chemical physical change Identify a
... 2. As an ion is formed, the mass number a. Increases b. Decreases c. Remains the same 3. If a neutral atom loses two electrons, the charge of the ion formed is a. -2 b. -1 c. 0 d. +1 e. +2 4. Compared to a proton, an electron a. Is smaller with the same charge b. Is larger with same charge c. Is sma ...
... 2. As an ion is formed, the mass number a. Increases b. Decreases c. Remains the same 3. If a neutral atom loses two electrons, the charge of the ion formed is a. -2 b. -1 c. 0 d. +1 e. +2 4. Compared to a proton, an electron a. Is smaller with the same charge b. Is larger with same charge c. Is sma ...
Trends in the Periodic Table
... movement of the subatomic particles, specifically electrons? • A: Temperature does not have enough energy to affect subatomic particle movement, only molecule movement. ...
... movement of the subatomic particles, specifically electrons? • A: Temperature does not have enough energy to affect subatomic particle movement, only molecule movement. ...
Thursday, 1/29/09 - Liberty Union High School District
... from a metal when light shines on metal •Light of minimum frequency was required to eject electron from metal •Problem-wave theory of light predicted light of any frequency could eject electron ...
... from a metal when light shines on metal •Light of minimum frequency was required to eject electron from metal •Problem-wave theory of light predicted light of any frequency could eject electron ...
Chemistry 2000 Review: quantum mechanics of
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
ionization 12.3.1
... bring about electron ionization. Fast atom bombardment ionization This term refers to the ionization of any species by causing interaction of the sample (which may be dissolved in a solvent matrix) and a beam of neutral atoms having a high translational energy. (See also secondary ionization). Field ...
... bring about electron ionization. Fast atom bombardment ionization This term refers to the ionization of any species by causing interaction of the sample (which may be dissolved in a solvent matrix) and a beam of neutral atoms having a high translational energy. (See also secondary ionization). Field ...
Document
... determines the angular shape of the orbital. ℓ’s have letter equivalents: ℓ = 0 (s), 1 (p), 2 (d), 3 (f), 4 (g), etc. ℓ = 0 ··· n-1 ...
... determines the angular shape of the orbital. ℓ’s have letter equivalents: ℓ = 0 (s), 1 (p), 2 (d), 3 (f), 4 (g), etc. ℓ = 0 ··· n-1 ...
2·QUIZLET VOCABULARY: Quantum Numbers Study online at
... 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) ...
... 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.