Chemical Reactions
... What is a chemical reaction? • A chemical reaction is the process by which the atoms of one or more substances are rearranged to form ...
... What is a chemical reaction? • A chemical reaction is the process by which the atoms of one or more substances are rearranged to form ...
Test 4 Review - Ralph C. Mahar
... The metal must be above (more active than) the ion for it to be a spontaneous reaction. ...
... The metal must be above (more active than) the ion for it to be a spontaneous reaction. ...
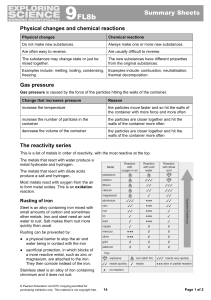
9F Reactivity - Parrs Wood High School
... These substances contain hydrogen and carbon only. They burn in a plentiful supply of air to form carbon dioxide and water: hydrocarbon + oxygen → carbon dioxide + water The test for oxygen is that it relights a glowing splint. An input of energy from a flame or spark is needed to start the combusti ...
... These substances contain hydrogen and carbon only. They burn in a plentiful supply of air to form carbon dioxide and water: hydrocarbon + oxygen → carbon dioxide + water The test for oxygen is that it relights a glowing splint. An input of energy from a flame or spark is needed to start the combusti ...
Name: Chemistry Honors Date: Period: ____ Reduction/Oxidation
... As you have learned, chemical reactions can be either endothermic or exothermic. This energy does not only have to be in the form of heat energy; it can also be electricity. These reactions can also occur spontaneously or non-spontaneously. In the case of any two metals on the activity series, the m ...
... As you have learned, chemical reactions can be either endothermic or exothermic. This energy does not only have to be in the form of heat energy; it can also be electricity. These reactions can also occur spontaneously or non-spontaneously. In the case of any two metals on the activity series, the m ...
ExamView - Untitled.tst
... b. stays the same. d. varies. ____ 11. A state of matter that is not a fluid is a. water. c. liquid. b. gas. d. solid. ____ 12. Dalton’s atomic theory was accepted because a. there was evidence to support it. b. Democritus said that it was correct. c. Dalton invented the electron microscope. d. Dalt ...
... b. stays the same. d. varies. ____ 11. A state of matter that is not a fluid is a. water. c. liquid. b. gas. d. solid. ____ 12. Dalton’s atomic theory was accepted because a. there was evidence to support it. b. Democritus said that it was correct. c. Dalton invented the electron microscope. d. Dalt ...
Types of Chemical Reactions
... couples. A and switch boyfriends, so is now going out with X and A is now going out with . combustion: a special kind of reaction in which a hydrocarbon (a compound containing carbon and hydrogen) reacts with O2 (burns, or “combusts”) to form CO2 and H2O. For example: C3H8 ...
... couples. A and switch boyfriends, so is now going out with X and A is now going out with . combustion: a special kind of reaction in which a hydrocarbon (a compound containing carbon and hydrogen) reacts with O2 (burns, or “combusts”) to form CO2 and H2O. For example: C3H8 ...
Chemistry 212 Name:
... oxidation state (+1, 3, 5, & 7). They all exist as colored diatomic molecules. ...
... oxidation state (+1, 3, 5, & 7). They all exist as colored diatomic molecules. ...
112- Unit I -Electrochem -pdf
... 1) a) The more positive E° red , the greater the tendency for the substance to be reduced. b) The substance is considered to be a strong oxidizing agent as the value of E° red becomes more positive. c) The more negative E° red , the weaker the tendency for the substance to be reduced. Actually, the ...
... 1) a) The more positive E° red , the greater the tendency for the substance to be reduced. b) The substance is considered to be a strong oxidizing agent as the value of E° red becomes more positive. c) The more negative E° red , the weaker the tendency for the substance to be reduced. Actually, the ...
Chemical Reactions
... • Lavoisier is known as the Father of Modern Chemistry for this work along with the work he did on types of reactions • Wrote a book called “Elements of Chemistry” in 1790 • He developed the nomenclature we use today to describe chemical compounds and reactions. ...
... • Lavoisier is known as the Father of Modern Chemistry for this work along with the work he did on types of reactions • Wrote a book called “Elements of Chemistry” in 1790 • He developed the nomenclature we use today to describe chemical compounds and reactions. ...
File
... Cations (+) and Anions (-): the cations will be attracted to the negative electrode & the anions will be attracted to the positive electrode. This movement sets up an electric current that is equivalent to the flow of electrons along a metal wire. ...
... Cations (+) and Anions (-): the cations will be attracted to the negative electrode & the anions will be attracted to the positive electrode. This movement sets up an electric current that is equivalent to the flow of electrons along a metal wire. ...
CHEMISTRY FINAL EXAM REVIEW SHEET
... Hydrogen is usually +1. Oxygen is usually –2. In a compound, the more electronegative element is given an oxidation number equal to its usual ionic charge. The sum of the oxidation numbers must equal the overall charge on the compound or ion. ...
... Hydrogen is usually +1. Oxygen is usually –2. In a compound, the more electronegative element is given an oxidation number equal to its usual ionic charge. The sum of the oxidation numbers must equal the overall charge on the compound or ion. ...
The five main types of redox reactions are combination
... are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always transferred between species. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more compl ...
... are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always transferred between species. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more compl ...
Chem 400 Chem 150 REVIEW SHEET Amanda R
... o Electron Affinity/Ionization Energy and electronegativity increases going up and to the right Types of Bonds – must know which bond types can form and how o Covalent o Ionic o Molecular o Bond order # of bonding e- - # of antibonding e-/2 Stoichiometry – must be able to balance reactions for any u ...
... o Electron Affinity/Ionization Energy and electronegativity increases going up and to the right Types of Bonds – must know which bond types can form and how o Covalent o Ionic o Molecular o Bond order # of bonding e- - # of antibonding e-/2 Stoichiometry – must be able to balance reactions for any u ...
1 - Intro to Electrochemistry
... A redox reaction is one where one substance is _______________ while another substance is simultaneously _______________ ...
... A redox reaction is one where one substance is _______________ while another substance is simultaneously _______________ ...
Chapter 4 Outline
... 1) If we mix 28.0 ml of 0.25 M HNO3 with 26.0 ml of 0.320 M Ba(OH)2 , calculate the moles of water formed in the reaction. Also, calculate the concentration of H + or OH - ions in excess after the reaction is complete. ...
... 1) If we mix 28.0 ml of 0.25 M HNO3 with 26.0 ml of 0.320 M Ba(OH)2 , calculate the moles of water formed in the reaction. Also, calculate the concentration of H + or OH - ions in excess after the reaction is complete. ...
CHAPTER 8
... Kb, pKa, pKb, pH of salt solutions, acidity of binary and oxyacids. 7. Aqueous ionic equilibrium: neutralization reactions, determining pH after addition of acid/base at various times, buffers, Henderson-Hasselbalch equation, titration curves, polyprotic acids/bases, solubility products for ionic co ...
... Kb, pKa, pKb, pH of salt solutions, acidity of binary and oxyacids. 7. Aqueous ionic equilibrium: neutralization reactions, determining pH after addition of acid/base at various times, buffers, Henderson-Hasselbalch equation, titration curves, polyprotic acids/bases, solubility products for ionic co ...
C1a - Mr Corfe
... EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical reaction where a single compound breaks up into two or more simpler compounds or elements when heated DEHYDRATION – chemical reaction that involves the loss of water from the reacting mol ...
... EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical reaction where a single compound breaks up into two or more simpler compounds or elements when heated DEHYDRATION – chemical reaction that involves the loss of water from the reacting mol ...
SNC1D Exam Review
... e) grounding f) voltage g) current h) resistance i) energy j) power k) potential difference l) electrolytic cell 2. Describe the law of electric charges and how it can be used to determine the charge on an object. 3. The electrostatic series is a great resource. a) What is it? b) What can it be used ...
... e) grounding f) voltage g) current h) resistance i) energy j) power k) potential difference l) electrolytic cell 2. Describe the law of electric charges and how it can be used to determine the charge on an object. 3. The electrostatic series is a great resource. a) What is it? b) What can it be used ...
Chemical Reactions, Chemical Equations, Electricity
... Electricity – an electric current, a form of energy. Current – a continuous flow of electrical charges through a material. Conductor – material that allows electrons move easily and freely forming an electric current. Insulator – material that does not allow electrical charges or electricity to flow ...
... Electricity – an electric current, a form of energy. Current – a continuous flow of electrical charges through a material. Conductor – material that allows electrons move easily and freely forming an electric current. Insulator – material that does not allow electrical charges or electricity to flow ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.