Redox Reactions and Electrochemistry
... different concentrations. The driving force (i.e. the EMF) is provided by the difference in concentrations. ...
... different concentrations. The driving force (i.e. the EMF) is provided by the difference in concentrations. ...
Redox Reactions: Transferring Electrons
... If a substance is losing the electron it is being oxidized If a substance is gaining, it is being reduced. ...
... If a substance is losing the electron it is being oxidized If a substance is gaining, it is being reduced. ...
Unit 1 Inorganic Flashcards
... surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
... surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
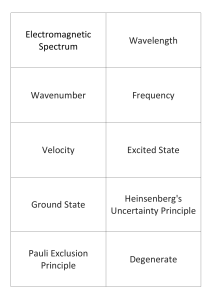
Electromagnetic Spectrum Wavelength Wavenumber Frequency
... surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
... surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
HOMEWORK : CHAPTER 20
... 20.50 The pressure of gaseous Al2Cl6 increases more rapidly with temperature than predicted by the ideal gas equation even though Al2Cl6 behaves like an ideal gas. Explain. 20.52 Explain the change in bonding when Al2Cl6 dissociates to form AlCl3 in the gas phase. 20.54 When 1.164 g of a certain met ...
... 20.50 The pressure of gaseous Al2Cl6 increases more rapidly with temperature than predicted by the ideal gas equation even though Al2Cl6 behaves like an ideal gas. Explain. 20.52 Explain the change in bonding when Al2Cl6 dissociates to form AlCl3 in the gas phase. 20.54 When 1.164 g of a certain met ...
The only sure evidence that a chemical reaction has occured is
... 13. Which reaction requires a continuous supply of energy in order to continue? ...
... 13. Which reaction requires a continuous supply of energy in order to continue? ...
PowerPoint Overview for Introduction
... and teeth. Ironically, calcium's most important role is in bodily functions, such as muscle contraction and protein regulation. In fact, the body will actually pull calcium from bones (causing problems like osteoporosis) if there's not enough of the element in a person's diet. ...
... and teeth. Ironically, calcium's most important role is in bodily functions, such as muscle contraction and protein regulation. In fact, the body will actually pull calcium from bones (causing problems like osteoporosis) if there's not enough of the element in a person's diet. ...
112 ex i lec outline
... light shines on metals, electrons could be ejected from the surface of the metals. For each metal there is a minimum frequency of light required to cause an electron to be released. Planck’s idea of energy quanta with the notion that light could be described not only as having wave-like properties b ...
... light shines on metals, electrons could be ejected from the surface of the metals. For each metal there is a minimum frequency of light required to cause an electron to be released. Planck’s idea of energy quanta with the notion that light could be described not only as having wave-like properties b ...
blood - SCH4U1-02-2010
... - The ideal pH level for blood is 7.35-7.45 - The higher the pH of a solution, the more electrical resistance that solution holds. - If pH level is either alkaline or acid, you will become ill, perhaps even die. 6. Electrolyte -Dietary Electrolytes and Blood Pressure. Electrolyte is a "medical/scien ...
... - The ideal pH level for blood is 7.35-7.45 - The higher the pH of a solution, the more electrical resistance that solution holds. - If pH level is either alkaline or acid, you will become ill, perhaps even die. 6. Electrolyte -Dietary Electrolytes and Blood Pressure. Electrolyte is a "medical/scien ...
What are reactions? - UTLNET Secure Site
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
What are reactions?
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
AP Ch. 20 Notes (2005)
... • If we examine the oxidation state of zinc, we see that zinc started out at zero and ended up at +2…it lost 2 electrons. − The process in which a substance increases its oxidation state (by losing electrons) is called “oxidation.” • If we examine the oxidation state of hydrogen, we see that hydroge ...
... • If we examine the oxidation state of zinc, we see that zinc started out at zero and ended up at +2…it lost 2 electrons. − The process in which a substance increases its oxidation state (by losing electrons) is called “oxidation.” • If we examine the oxidation state of hydrogen, we see that hydroge ...
Redox Reactions and Electrochemistry
... different concentrations. The driving force (i.e. the EMF) is provided by the difference in concentrations. ...
... different concentrations. The driving force (i.e. the EMF) is provided by the difference in concentrations. ...
Production of Materials by Jimmy Huang
... Chapter 3 - The Availability of Other Resources from Renewable Resources Ethanol As A Source of Ethylene Ethanol has the structural formula CH3-CH2-OH, meaning it is an alkane with one H atom replaced by the OH functional group. It is the most widely used alcohol, which is a family of carbon compou ...
... Chapter 3 - The Availability of Other Resources from Renewable Resources Ethanol As A Source of Ethylene Ethanol has the structural formula CH3-CH2-OH, meaning it is an alkane with one H atom replaced by the OH functional group. It is the most widely used alcohol, which is a family of carbon compou ...
File
... Inside the battery itself, a chemical reaction produces the electrons. Electrons flow from the battery into a wire, and must travel from the negative to the positive terminal (anode to cathode) for the chemical reaction to take place. That is why a battery can sit on a shelf for a year and still ha ...
... Inside the battery itself, a chemical reaction produces the electrons. Electrons flow from the battery into a wire, and must travel from the negative to the positive terminal (anode to cathode) for the chemical reaction to take place. That is why a battery can sit on a shelf for a year and still ha ...
1.5.16(Chem) - mrcarlsonschemistryclass
... Compounds • Atoms bonded together with an IONIC bond are called ionic compounds. • An ionic bond is a METAL bonded with a NONMETAL. • Draw the crystal lattice structure for sodium chloride: ...
... Compounds • Atoms bonded together with an IONIC bond are called ionic compounds. • An ionic bond is a METAL bonded with a NONMETAL. • Draw the crystal lattice structure for sodium chloride: ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.