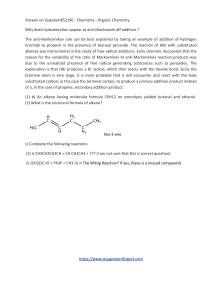
Answer on Question#52196 - Chemistry
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
Name Date ______ Period - Staff Websites | Blogs
... 1. Identify what the formation of all macromolecules from their specific monomers has in common. 2. Cellulose and starch are both made of repeating units of glucose. Discuss what is different about their structure that gives them such different chemical properties and uses. 3. Lipids do not have a c ...
... 1. Identify what the formation of all macromolecules from their specific monomers has in common. 2. Cellulose and starch are both made of repeating units of glucose. Discuss what is different about their structure that gives them such different chemical properties and uses. 3. Lipids do not have a c ...
Learning Guide: Water and Biomolecules (cont.) Bill Activity #17 To
... o Describe how Stanley Miller bring the abiotic synthesis of organic compounds into the context of evolution. Carbon atoms can form diverse molecules by bonding to four other atoms. o Make an electron distribution diagram of carbon. List the number of valence electrons, number of bonds and types o ...
... o Describe how Stanley Miller bring the abiotic synthesis of organic compounds into the context of evolution. Carbon atoms can form diverse molecules by bonding to four other atoms. o Make an electron distribution diagram of carbon. List the number of valence electrons, number of bonds and types o ...
Chemistry of Organic Molecules
... Chemistry of Organic Molecules 3.1 Organic molecules overview ...
... Chemistry of Organic Molecules 3.1 Organic molecules overview ...
Structure and Shape - Chapter 12 Valence Shell Electron Pair
... Organic Chemistry Organic chemistry - the chemistry of carbon compounds. Hydrocarbons - compounds made up only of carbon and hydrogen. Alkanes - one type of hydrocarbon molecule which consist of carbon atoms attached to each other, forming chains. ...
... Organic Chemistry Organic chemistry - the chemistry of carbon compounds. Hydrocarbons - compounds made up only of carbon and hydrogen. Alkanes - one type of hydrocarbon molecule which consist of carbon atoms attached to each other, forming chains. ...
Name Date ______ Period _____
... Why study carbon? ________________________________________________________________________ ...
... Why study carbon? ________________________________________________________________________ ...
What is an addition reaction
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
Focus
... group • functional group • A group of atoms bonded to a carbon of an organic compound • Imparts a specific chemical property such as polarity or acidity ...
... group • functional group • A group of atoms bonded to a carbon of an organic compound • Imparts a specific chemical property such as polarity or acidity ...
The following list of topics for an AP Chemistry course is intended to
... 2. Atomic masses; determination by chemical and physical means 3. Atomic number and mass number; isotopes 4. Electron energy levels: atomic spectra, quantum numbers, atomic orbitals 5. Periodic relationships including, for example, atomic radii, ionization energies, electron affinities, oxidation st ...
... 2. Atomic masses; determination by chemical and physical means 3. Atomic number and mass number; isotopes 4. Electron energy levels: atomic spectra, quantum numbers, atomic orbitals 5. Periodic relationships including, for example, atomic radii, ionization energies, electron affinities, oxidation st ...
Chemistry Honors Study Guide – Organic Chemistry You should be
... □ Write general, molecular, structural, condensed structural, carbon skeleton, and line-angle formulas of organic molecules ...
... □ Write general, molecular, structural, condensed structural, carbon skeleton, and line-angle formulas of organic molecules ...
Chapter 19
... The physical properties of organic compounds can be predicted from the molecular structure. ...
... The physical properties of organic compounds can be predicted from the molecular structure. ...
Test 12
... REGENTS & HONORS CHEMISTRY TEST #12 Key Terms and Important Stuff to Know ORGANIC CHEMISTRY Organic molecules must contain carbon and hydrogen Covalently bonded molecules Properties of organic compounds Most non-polar Most not soluble in water (if non-polar) Low M.P./B.P. due to weak intermolecular ...
... REGENTS & HONORS CHEMISTRY TEST #12 Key Terms and Important Stuff to Know ORGANIC CHEMISTRY Organic molecules must contain carbon and hydrogen Covalently bonded molecules Properties of organic compounds Most non-polar Most not soluble in water (if non-polar) Low M.P./B.P. due to weak intermolecular ...
Project Details PPT
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
File - Mrs. LeCompte
... FUNCTIONAL GROUP = a specific combination of bonded atoms that always reacts in the same way, regardless of the C-skeleton it is bonded to Are often the chemically reactive part of the molecule Determine the unique chemical properties of the organic molecules in which they occur Please note that ...
... FUNCTIONAL GROUP = a specific combination of bonded atoms that always reacts in the same way, regardless of the C-skeleton it is bonded to Are often the chemically reactive part of the molecule Determine the unique chemical properties of the organic molecules in which they occur Please note that ...
Organic Tutorial 1st Year MT03
... Peter Sykes,“A Guidebook to Mechanism in Organic Chemistry”, and Eames & Peach “Stereochemistry at a Glance”. Notes and Questions a) Summary on not more than 6 sides. This should outline the possible mechanisms and the evidence on which they are based, in particular the evidence for inversion during ...
... Peter Sykes,“A Guidebook to Mechanism in Organic Chemistry”, and Eames & Peach “Stereochemistry at a Glance”. Notes and Questions a) Summary on not more than 6 sides. This should outline the possible mechanisms and the evidence on which they are based, in particular the evidence for inversion during ...