Bioenergetics Study Guide Bioenergetics – Overview Use the
... Electron Transport Chain - What is it? - How does it work? (General overview – You will not need to have all the steps memorized, but be able to describe the steps if shown a diagram.) - What is used during glycolysis? - What is produced? - How are the electron transport chain and the citric acid cy ...
... Electron Transport Chain - What is it? - How does it work? (General overview – You will not need to have all the steps memorized, but be able to describe the steps if shown a diagram.) - What is used during glycolysis? - What is produced? - How are the electron transport chain and the citric acid cy ...
Amino Acid Catabolism
... • NADPH dependent • Chemotherapy dtarget – DHF analogs such as methotrexate ...
... • NADPH dependent • Chemotherapy dtarget – DHF analogs such as methotrexate ...
Appendices 1-5
... 1) Cytochrome b-245 polypeptide (Cyba), a membrane bound superoxide generator that belongs to the p22phox family. 2) Neutrophil cytosolic factor 4, p40phox (Ncf4), through interaction with an SH3 domain is responsible for the down-regulation of NADH-oxidase, and associates primarily with p67phox t ...
... 1) Cytochrome b-245 polypeptide (Cyba), a membrane bound superoxide generator that belongs to the p22phox family. 2) Neutrophil cytosolic factor 4, p40phox (Ncf4), through interaction with an SH3 domain is responsible for the down-regulation of NADH-oxidase, and associates primarily with p67phox t ...
Chapter 5:Bioenergetics and oxidative phosphorylation Q1: why is
... Q3: Are NADH & FADH2 Produced in the mitochondria only? Q4: why does FADH2 produce 2ATP while NADH produce 3ATP? Q5: what are the site-specific inhibitors of the electron transport chain? Q6: Explain why NADH is oxidized by FMN? Q7: How is electron transport chain is coupled to oxidative phosphoryla ...
... Q3: Are NADH & FADH2 Produced in the mitochondria only? Q4: why does FADH2 produce 2ATP while NADH produce 3ATP? Q5: what are the site-specific inhibitors of the electron transport chain? Q6: Explain why NADH is oxidized by FMN? Q7: How is electron transport chain is coupled to oxidative phosphoryla ...
Bio130_MidtermReviewPart3
... based on three catabolic pathways that convert glucose to CO2 and gives off energy • Aerobic respiration – glycolysis, the Kreb’s cycle, respiratory chain • Anaerobic respiration – glycolysis, the Kreb’s cycle, respiratory chain; molecular oxygen is not the final electron acceptor • Fermentation – g ...
... based on three catabolic pathways that convert glucose to CO2 and gives off energy • Aerobic respiration – glycolysis, the Kreb’s cycle, respiratory chain • Anaerobic respiration – glycolysis, the Kreb’s cycle, respiratory chain; molecular oxygen is not the final electron acceptor • Fermentation – g ...
- Angelo State University
... • Lipids improve the texture of foods, absorb and retain flavors, and are digested more slowly than other foods, prolong the feeling of satiety (satisfaction and fullness after a meal). • Linoleic and linolenic acids are essential fatty acids because they cannot be synthesized in the body, and must ...
... • Lipids improve the texture of foods, absorb and retain flavors, and are digested more slowly than other foods, prolong the feeling of satiety (satisfaction and fullness after a meal). • Linoleic and linolenic acids are essential fatty acids because they cannot be synthesized in the body, and must ...
Citric acid cycle
... • Before the citric acid cycle can begin – Pyruvate must first be converted to acetyl CoA, which links the cycle to glycolysis – Fully oxidized carboxyl group is removed as a CO2 – The remaining two-carbon fragment is oxidized to acetate. The extracted electrons are transferred to NAD+, forming NAD ...
... • Before the citric acid cycle can begin – Pyruvate must first be converted to acetyl CoA, which links the cycle to glycolysis – Fully oxidized carboxyl group is removed as a CO2 – The remaining two-carbon fragment is oxidized to acetate. The extracted electrons are transferred to NAD+, forming NAD ...
ATP-binding site as a further application of neural network
... of ATP, the later being a small molecules. 3. ATP shows smallest propensity for Trp (prop=0.5) residues in contrast to sugars (prop> 3.0), other ligands (prop> 2.0) and DNA (prop close to 1, almost neutral). This could be partly because Trp is typically a buried residue and only those Trp side chain ...
... of ATP, the later being a small molecules. 3. ATP shows smallest propensity for Trp (prop=0.5) residues in contrast to sugars (prop> 3.0), other ligands (prop> 2.0) and DNA (prop close to 1, almost neutral). This could be partly because Trp is typically a buried residue and only those Trp side chain ...
Student PPT Notes
... Some enzymes only synthesized at ________________________in organisms life (65% of ppl produce less lactase as they age) inactive forms of enzymes only become ________________when needed (i.e. protein digesting enzymes pepsin & trypsin) ...
... Some enzymes only synthesized at ________________________in organisms life (65% of ppl produce less lactase as they age) inactive forms of enzymes only become ________________when needed (i.e. protein digesting enzymes pepsin & trypsin) ...
Chapter 16 The Citric Acid Cycle
... 7. Conversion of 1 mol of acetyl-CoA to 2 mol of CO2 via the citric acid cycle results in the net production of: A) 1 mol of citrate. B) 1 mol of FADH2. C) 1 mol of NADH. D) 1 mol of oxaloacetate. E) 7 mol of ATP. 8. The oxidative decarboxylation of α-ketoglutarate proceeds by means of multistep re ...
... 7. Conversion of 1 mol of acetyl-CoA to 2 mol of CO2 via the citric acid cycle results in the net production of: A) 1 mol of citrate. B) 1 mol of FADH2. C) 1 mol of NADH. D) 1 mol of oxaloacetate. E) 7 mol of ATP. 8. The oxidative decarboxylation of α-ketoglutarate proceeds by means of multistep re ...
3.DCP I Year BCP Metabolism Notes
... 2. Anabolism : Formation of the new molecule. e.g. Amino acids by polypeptide bonds forms proteins. ...
... 2. Anabolism : Formation of the new molecule. e.g. Amino acids by polypeptide bonds forms proteins. ...
2004 Lec 42-43: Nucleotide Metabolism
... Allosteric feedback inhibition of PRPP-Gln amidotransferase Salvage of free bases Nucleosides and nucleotides spontaneously hydrolyze N-b-glycosidic bond free base released catabolized to urate De novo synthesis of purines minimized by 1-step conversion back to nucleoside-5’P ...
... Allosteric feedback inhibition of PRPP-Gln amidotransferase Salvage of free bases Nucleosides and nucleotides spontaneously hydrolyze N-b-glycosidic bond free base released catabolized to urate De novo synthesis of purines minimized by 1-step conversion back to nucleoside-5’P ...
Chapter 27-28 - Bakersfield College
... The rate of the citric acid cycle depends on the body’s need for energy. When energy demands are high and ATP is low → the cycle is activated. When energy demands are low and NADH is high → the cycle is inhibited. ...
... The rate of the citric acid cycle depends on the body’s need for energy. When energy demands are high and ATP is low → the cycle is activated. When energy demands are low and NADH is high → the cycle is inhibited. ...
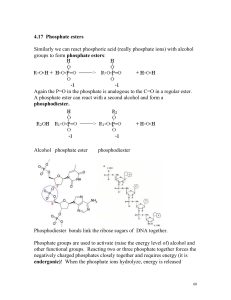
4.17 Phosphate esters Similarly we can react phosphoric acid (really
... A phosphate ester can react with a second alcohol and form a phosphodiester. ...
... A phosphate ester can react with a second alcohol and form a phosphodiester. ...
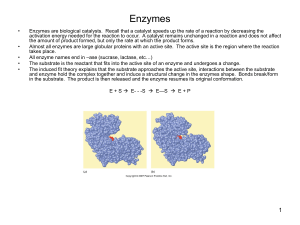
Enzymes
... Enzymes are biological catalysts. Recall that a catalyst speeds up the rate of a reaction by decreasing the activation energy needed for the reaction to occur. A catalyst remains unchanged in a reaction and does not affect the amount of product formed, but only the rate at which the product forms. A ...
... Enzymes are biological catalysts. Recall that a catalyst speeds up the rate of a reaction by decreasing the activation energy needed for the reaction to occur. A catalyst remains unchanged in a reaction and does not affect the amount of product formed, but only the rate at which the product forms. A ...
Transamination, Deamination,urea cycle
... • first two reactions leading to the synthesis of urea occur in the mitochondria, whereas the remaining cycle enzymes are located in the cytosol • One nitrogen of the urea molecule is supplied by free ammonia, and the other nitrogen by aspartate ...
... • first two reactions leading to the synthesis of urea occur in the mitochondria, whereas the remaining cycle enzymes are located in the cytosol • One nitrogen of the urea molecule is supplied by free ammonia, and the other nitrogen by aspartate ...
Cell transport with the environment
... membrane by using energy (ATP). Particles such as sodium and potassium ions often need the help of ATP to pass through the cell membrane. Active transport occurs when the particle is moved against the gradient. Low concentration ...
... membrane by using energy (ATP). Particles such as sodium and potassium ions often need the help of ATP to pass through the cell membrane. Active transport occurs when the particle is moved against the gradient. Low concentration ...
A CBS domain-containing pyrophosphatase of Moorella
... PPi (inorganic pyrophosphate) is produced in vast amounts by biosynthetic reactions, such as protein, RNA and DNA synthesis, and its concentration affects the equilibria of these reactions [1]. In addition, PPi regulates many other cellular processes, including calcification, cell proliferation and ...
... PPi (inorganic pyrophosphate) is produced in vast amounts by biosynthetic reactions, such as protein, RNA and DNA synthesis, and its concentration affects the equilibria of these reactions [1]. In addition, PPi regulates many other cellular processes, including calcification, cell proliferation and ...
Lipid Metabolism: Power Point presentation
... • Longer fatty acids are produced from palmitate using special enzymes. • Unsaturated cis bonds are incorporated into a 10-carbon fatty acid that is elongated further. ...
... • Longer fatty acids are produced from palmitate using special enzymes. • Unsaturated cis bonds are incorporated into a 10-carbon fatty acid that is elongated further. ...
ADP
... ③ The key points of TAC For each cycle of TAC, – One molecule of acetyl CoA is consumed – Undergo through four times of dehydrogenation, two times of decarboxylation, one time of substrate level phosphorylation – Yield one molecule of FADH2, three molecules of NADH+H+, two molecules of CO2, one mol ...
... ③ The key points of TAC For each cycle of TAC, – One molecule of acetyl CoA is consumed – Undergo through four times of dehydrogenation, two times of decarboxylation, one time of substrate level phosphorylation – Yield one molecule of FADH2, three molecules of NADH+H+, two molecules of CO2, one mol ...
AdvLec10_WebCT
... ATP used up to ph’late fructose cellular energy is reduced phosphate ‘trapped’ in fructose 1 P all of the above ...
... ATP used up to ph’late fructose cellular energy is reduced phosphate ‘trapped’ in fructose 1 P all of the above ...
NHM 555 - Pennington Biomedical Research Center
... If there is plenty of oxygen available in muscles (aerobic state) and the physical activity is of moderate to low intensity (jogging or distance swimming), then the bulk of pyruvate produced by glycolysis is shuttled to the mitochondria and further metabolized into carbon dioxide and water in a seri ...
... If there is plenty of oxygen available in muscles (aerobic state) and the physical activity is of moderate to low intensity (jogging or distance swimming), then the bulk of pyruvate produced by glycolysis is shuttled to the mitochondria and further metabolized into carbon dioxide and water in a seri ...
A Novel Recombinant Plasma Membrane
... ATP release triggered by receptor-directed stimuli, we challenged the HEK293-pmeLUC cells with various agonists of G protein-coupled receptors (e.g., carbachol, histamine, bradykinin), obtaining negligible ATP release (unpublished data). Early experiments from our and other laboratories had previous ...
... ATP release triggered by receptor-directed stimuli, we challenged the HEK293-pmeLUC cells with various agonists of G protein-coupled receptors (e.g., carbachol, histamine, bradykinin), obtaining negligible ATP release (unpublished data). Early experiments from our and other laboratories had previous ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.