Fermentation
... Fermentation Under anaerobic conditions, fermentation follows glycolysis. During fermentation, cells convert NADH produced by glycolysis back into the electron carrier NAD+, which allows glycolysis to continue producing ATP. ...
... Fermentation Under anaerobic conditions, fermentation follows glycolysis. During fermentation, cells convert NADH produced by glycolysis back into the electron carrier NAD+, which allows glycolysis to continue producing ATP. ...
gluconeogenesis
... The two phases of glycolysis. For each molecule of glucose that passes through the preparatory phase (a), two molecules of glyceraldehyde3-phosphate are formed; both pass through the payoff phase (b). Pyruvate is the end product of the second phase under aerobic conditions, but under anaerobic ...
... The two phases of glycolysis. For each molecule of glucose that passes through the preparatory phase (a), two molecules of glyceraldehyde3-phosphate are formed; both pass through the payoff phase (b). Pyruvate is the end product of the second phase under aerobic conditions, but under anaerobic ...
electron transport chain
... • Each two-carbon acetyl group combines with a four-carbon compound • Two CO2 molecules are removed • Energy captured as one ATP, three NADH, and one FADH2 per acetyl group (These will act as energy carriers for the electron transport chain) **So… 2 acetyl groups yields 4 CO2, 6 NADH, 2 FADH2, 2 ATP ...
... • Each two-carbon acetyl group combines with a four-carbon compound • Two CO2 molecules are removed • Energy captured as one ATP, three NADH, and one FADH2 per acetyl group (These will act as energy carriers for the electron transport chain) **So… 2 acetyl groups yields 4 CO2, 6 NADH, 2 FADH2, 2 ATP ...
Lecture 12-14 (Parker) - Department of Chemistry ::: CALTECH
... The Glycolytic pathway is tightly controlled The rate of conversion of glucose to pyruvate is tightly regulated. In glycolysis the reactions catalyzed by hexokinase, phosphofructokinase and pyruvate kinase are essentially irreversible making them targets for regulation. These enzymes become more ...
... The Glycolytic pathway is tightly controlled The rate of conversion of glucose to pyruvate is tightly regulated. In glycolysis the reactions catalyzed by hexokinase, phosphofructokinase and pyruvate kinase are essentially irreversible making them targets for regulation. These enzymes become more ...
AMPK_PhD
... the A8344G in the tRNALys gene in myoclonic epilepsy associated with ragged-red fibres (MERRF) The T8993G leads to neurogenic weakness, ataxia, and retinitis pigmentosa (NARP), ...
... the A8344G in the tRNALys gene in myoclonic epilepsy associated with ragged-red fibres (MERRF) The T8993G leads to neurogenic weakness, ataxia, and retinitis pigmentosa (NARP), ...
Chapter 6
... – The citric acid cycle: • Extracts the energy of sugar by breaking the acetic acid molecules all the way down to CO2 • Uses some of this energy to make ATP • Forms NADH and FADH2 Laua Coronado ...
... – The citric acid cycle: • Extracts the energy of sugar by breaking the acetic acid molecules all the way down to CO2 • Uses some of this energy to make ATP • Forms NADH and FADH2 Laua Coronado ...
Document
... Why is oxidative phosphorylation the most important mechanism for generating ATP? a. It requires less energy than other mechanisms. b. It requires fewer steps to produce ATP molecules. c. It produces more than 90% of ATP used by body cells. d. It allows the release of a tremendous amount of energy. ...
... Why is oxidative phosphorylation the most important mechanism for generating ATP? a. It requires less energy than other mechanisms. b. It requires fewer steps to produce ATP molecules. c. It produces more than 90% of ATP used by body cells. d. It allows the release of a tremendous amount of energy. ...
solute - Life Science Academy
... cellular work ATP- Adenosine Triphosphate ATP powers nearly all forms of cellular work The energy in an ATP molecule is in the bonds between its phosphate groups ATP is the pocketbook! ...
... cellular work ATP- Adenosine Triphosphate ATP powers nearly all forms of cellular work The energy in an ATP molecule is in the bonds between its phosphate groups ATP is the pocketbook! ...
GLUCONEOGENESIS, GLYCOGEN SYNTHESIS & DEGRADATION
... purposes in liver and muscle. The liver synthesizes glycogen after a carbohydrate meal and degrades it to free glucose during fasting. The glucose-6-phosphate from glycogen breakdown is cleaved to free glucose by glucose-6-phosphatase. The liver releases this glucose into the blood for use by ...
... purposes in liver and muscle. The liver synthesizes glycogen after a carbohydrate meal and degrades it to free glucose during fasting. The glucose-6-phosphate from glycogen breakdown is cleaved to free glucose by glucose-6-phosphatase. The liver releases this glucose into the blood for use by ...
Chapter 9
... 6) Where does glycolysis takes place? A) mitochondrial matrix B) mitochondrial outer membrane C) mitochondrial inner membrane D) mitochondrial intermembrane space E) cytosol Answer: E Topic: Concept 9.1 Skill: Knowledge/Comprehension 7) The ATP made during glycolysis is generated by A) substrate-lev ...
... 6) Where does glycolysis takes place? A) mitochondrial matrix B) mitochondrial outer membrane C) mitochondrial inner membrane D) mitochondrial intermembrane space E) cytosol Answer: E Topic: Concept 9.1 Skill: Knowledge/Comprehension 7) The ATP made during glycolysis is generated by A) substrate-lev ...
Types of Organic compounds
... and a hydroxyl group attached to the same carbon atom, resulting in new properties. Carboxyl groups frequently ionize, releasing the H from the hydroxyl group as a free proton (H+), with the remaining O carrying a negative charge. Molecules containing carboxyl groups are called carboxylic acids and ...
... and a hydroxyl group attached to the same carbon atom, resulting in new properties. Carboxyl groups frequently ionize, releasing the H from the hydroxyl group as a free proton (H+), with the remaining O carrying a negative charge. Molecules containing carboxyl groups are called carboxylic acids and ...
Muscles
... Substrate level phosphorylation: macroergic phosphate X~P + ADP ATP + second product ...
... Substrate level phosphorylation: macroergic phosphate X~P + ADP ATP + second product ...
Introduction to Cellular and Molecular Biology (BIOL 190)
... carbons, how many CO2 are produced, and how many NADH, FADH2, and ATP are generated 4. Understand that some energy from glucose is being carried in the NADH and FADH2 produced during glycolysis, the oxidation of pyruvate and the citric acid cycle 5. Know that the ATP produced so far are made by dire ...
... carbons, how many CO2 are produced, and how many NADH, FADH2, and ATP are generated 4. Understand that some energy from glucose is being carried in the NADH and FADH2 produced during glycolysis, the oxidation of pyruvate and the citric acid cycle 5. Know that the ATP produced so far are made by dire ...
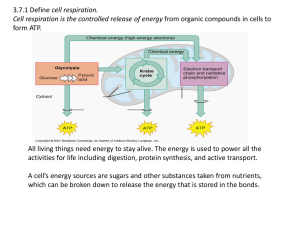
3.7:Cell Respiration Aerobic cell respiration: glucose
... All living things need energy to stay alive. The energy is used to power all the activities for life including digestion, protein synthesis, and active transport. A cell’s energy sources are sugars and other substances taken from nutrients, which can be broken down to release the energy that is stor ...
... All living things need energy to stay alive. The energy is used to power all the activities for life including digestion, protein synthesis, and active transport. A cell’s energy sources are sugars and other substances taken from nutrients, which can be broken down to release the energy that is stor ...
The Central Role of Acetyl-CoA
... • Adenosine triphosphate ATP – the battery of life • Biological processes requiring energy use ATP • The accessible energy in ATP lies in the triphosphate ...
... • Adenosine triphosphate ATP – the battery of life • Biological processes requiring energy use ATP • The accessible energy in ATP lies in the triphosphate ...
ppt
... • Gal is a component of lactose (Glc + Gal) • Gal is absorbed by the same mechanism in enterocytes like Glc → liver • Gal is phosphorylated in liver to form Gal-1-P: Gal + ATP → Gal-1-P + ADP by enzyme galactokinase • Gal-1-P is converted to UDP-Gal: Gal-1-P + UTP → UDP-Gal + PPi by uridyltransferas ...
... • Gal is a component of lactose (Glc + Gal) • Gal is absorbed by the same mechanism in enterocytes like Glc → liver • Gal is phosphorylated in liver to form Gal-1-P: Gal + ATP → Gal-1-P + ADP by enzyme galactokinase • Gal-1-P is converted to UDP-Gal: Gal-1-P + UTP → UDP-Gal + PPi by uridyltransferas ...
EXS202: HUMAN PHYSIOLOGY 1: LECTURE NOTES (SAMPLE)
... found in the muscles of vertebrates where its hydrolysis releases energy for muscular contraction. What is ATP? ATP is adenosine triphosphate. ATP is a nucleotide derived from adenosine that occurs in muscle tissue. ATP is the major source of energy for cellular reactions. ATP is our preferred energ ...
... found in the muscles of vertebrates where its hydrolysis releases energy for muscular contraction. What is ATP? ATP is adenosine triphosphate. ATP is a nucleotide derived from adenosine that occurs in muscle tissue. ATP is the major source of energy for cellular reactions. ATP is our preferred energ ...
Carbohydrate Metabolism Updated
... oxidized through this pathway. • Uronic acid pathway:Glucose is oxidized to glucuronic acid. • Galactose metabolism:Galactose is converted to glucose. • Fructose metabolism:Fructose is converted to glucose or metabolized in liver. ...
... oxidized through this pathway. • Uronic acid pathway:Glucose is oxidized to glucuronic acid. • Galactose metabolism:Galactose is converted to glucose. • Fructose metabolism:Fructose is converted to glucose or metabolized in liver. ...
Metabolic modeling and comparative biochemistry in glyoxylate cycle
... FADH2 oxidation (Buchanan et al., 2015). Since α carbon is esterified with coenzyme A, the β− 1 times, where n is the ...
... FADH2 oxidation (Buchanan et al., 2015). Since α carbon is esterified with coenzyme A, the β− 1 times, where n is the ...
1 - u.arizona.edu
... Inhibition by acidic conditions - during anaerobic carbohydrate metabolism in muscle, lactic acid is a product - excess production of acid lower intracellular pH; to prevent further decline of cell pH through production of more acid via metabolism elevated [H+] concentration inhibits PFK1 to sl ...
... Inhibition by acidic conditions - during anaerobic carbohydrate metabolism in muscle, lactic acid is a product - excess production of acid lower intracellular pH; to prevent further decline of cell pH through production of more acid via metabolism elevated [H+] concentration inhibits PFK1 to sl ...
Energetic Crosstalk Between Organelles
... loading. However, these experiments were unable to answer the question of the efficacy of the mitochondrially supported SR load, because high ADP concentrations are known to inhibit or reverse the SR calcium pump. We thus replaced external ADP with ATP in the loading solutions. Under these condition ...
... loading. However, these experiments were unable to answer the question of the efficacy of the mitochondrially supported SR load, because high ADP concentrations are known to inhibit or reverse the SR calcium pump. We thus replaced external ADP with ATP in the loading solutions. Under these condition ...
Energetic Crosstalk Between Organelles
... loading. However, these experiments were unable to answer the question of the efficacy of the mitochondrially supported SR load, because high ADP concentrations are known to inhibit or reverse the SR calcium pump. We thus replaced external ADP with ATP in the loading solutions. Under these condition ...
... loading. However, these experiments were unable to answer the question of the efficacy of the mitochondrially supported SR load, because high ADP concentrations are known to inhibit or reverse the SR calcium pump. We thus replaced external ADP with ATP in the loading solutions. Under these condition ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.