4.2 Respiration – Page 1 S. Preston 1 From the
... made available for all the active processes within a cell. This achieved by the controlled enzymatic breakdown of glucose, the energy released is stored in molecules of ATP (if the energy released from glucose proceeded in an uncontrolled manner it would produce a temperature rise that would destroy ...
... made available for all the active processes within a cell. This achieved by the controlled enzymatic breakdown of glucose, the energy released is stored in molecules of ATP (if the energy released from glucose proceeded in an uncontrolled manner it would produce a temperature rise that would destroy ...
Chapter 26 - McGraw Hill Higher Education
... away as CO2 and exhaled. • Energy lost as heat, stored in 2 ATP, 8 reduced NADH, 2 FADH2 molecules of the matrix reactions and 2 NADH from glycolysis • Citric acid cycle is a source of substances for synthesis of fats and nonessential amino acids 26-49 ...
... away as CO2 and exhaled. • Energy lost as heat, stored in 2 ATP, 8 reduced NADH, 2 FADH2 molecules of the matrix reactions and 2 NADH from glycolysis • Citric acid cycle is a source of substances for synthesis of fats and nonessential amino acids 26-49 ...
General Biology 115 Summer 2014
... Oxygen cannot be reduced Electrons cannot flow to Complex III Less H+ will be pumped across the inner mitochondrial membrane B&C A, B & C ...
... Oxygen cannot be reduced Electrons cannot flow to Complex III Less H+ will be pumped across the inner mitochondrial membrane B&C A, B & C ...
Problem Set 2 (multiple choice) Biochemistry 3300 1. What classes
... 19. All of the following contribute to the large, negative, free-energy change upon hydrolysis of “high-energy” compounds except: a) electrostatic repulsion in the reactant. b) low activation energy of forward reaction. c) stabilization of products by extra resonance forms. d) stabilization of produ ...
... 19. All of the following contribute to the large, negative, free-energy change upon hydrolysis of “high-energy” compounds except: a) electrostatic repulsion in the reactant. b) low activation energy of forward reaction. c) stabilization of products by extra resonance forms. d) stabilization of produ ...
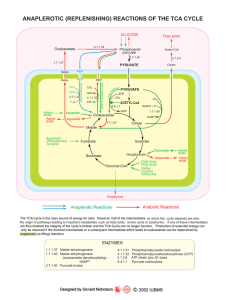
anaplerotic (replenishing) reactions of the tca cycle - Sigma
... The TCA Cycle is the main source of energy for cells. However, half of the intermediates on which the cycle depends are also the origin of pathways leading to important metabolites such as fatty acids , amino acids or porphyrins. If any of these intermediates are thus diverted the integrity of the c ...
... The TCA Cycle is the main source of energy for cells. However, half of the intermediates on which the cycle depends are also the origin of pathways leading to important metabolites such as fatty acids , amino acids or porphyrins. If any of these intermediates are thus diverted the integrity of the c ...
Bio AP chp 9 notes
... As hydrogen ions flow down their gradient, they cause the cylinder portion and attached rod of ATP synthase to rotate. ...
... As hydrogen ions flow down their gradient, they cause the cylinder portion and attached rod of ATP synthase to rotate. ...
Cell Respir/Ferm slide
... In this case study, students learn about the function of cellular respiration and the electron transport chain and what happens when that function is impaired. Students play the role of medical examiner as they analyze the autopsy results to determine the cause of the mysterious deaths of these seve ...
... In this case study, students learn about the function of cellular respiration and the electron transport chain and what happens when that function is impaired. Students play the role of medical examiner as they analyze the autopsy results to determine the cause of the mysterious deaths of these seve ...
How Do Enzymes Work?
... conformations (NACs) – NACs are precursors to reaction transition states ...
... conformations (NACs) – NACs are precursors to reaction transition states ...
AP Biology Photosynthesis – Part 3 Text reading 10.3 Important
... 2) The macromolecule Carbohydrate is an energy storage molecule that is intended for quick release of energy. 3) Carbon is an important molecule in making macromolecules. The primary source is from CO2 in air. I. Calvin Cycle (A.K.A Light Independent reaction) A. This part uses the ATP and NADPH, of ...
... 2) The macromolecule Carbohydrate is an energy storage molecule that is intended for quick release of energy. 3) Carbon is an important molecule in making macromolecules. The primary source is from CO2 in air. I. Calvin Cycle (A.K.A Light Independent reaction) A. This part uses the ATP and NADPH, of ...
Ab`s Simplistic Cell Biology Cell theory is a great example of
... In general we shall be talking about the complete metabolism of the fuel, glucose, in oxygen. However, most cells can keep running (at least for a while) when oxygen is not present, or when a circulatory system cannot deliver oxygen fast enough to keep pace with metabolic demands. Under these circum ...
... In general we shall be talking about the complete metabolism of the fuel, glucose, in oxygen. However, most cells can keep running (at least for a while) when oxygen is not present, or when a circulatory system cannot deliver oxygen fast enough to keep pace with metabolic demands. Under these circum ...
THE CITRIC ACID CYCLE
... • Compare to ATP phosphate hydrolysis at -30 kJ/mole • We preserve that energy by making GTP • This reaction utilizes a swinging histidine side chain to transfer the PO42- group from succinyl phosphate to ...
... • Compare to ATP phosphate hydrolysis at -30 kJ/mole • We preserve that energy by making GTP • This reaction utilizes a swinging histidine side chain to transfer the PO42- group from succinyl phosphate to ...
sheet#11
... Glycolysis is a universal pathway in all cell types. It occurs by the same mechanism and with similar enzymes. It is a pathway for generating ATP with or without the presence of oxygen (anaerobic). In addition to that, it produces important biosynthetic precursors for anabolic pathways; for example, ...
... Glycolysis is a universal pathway in all cell types. It occurs by the same mechanism and with similar enzymes. It is a pathway for generating ATP with or without the presence of oxygen (anaerobic). In addition to that, it produces important biosynthetic precursors for anabolic pathways; for example, ...
Iron-sulfur proteins
... • When cell receives a signal for apoptosis, one consequence is the permeability of the outer mitochondrial membrane will increase, allowing cytochrome c release. • The release of cytochrome c will activate caspase 9, which will initiate the protein ...
... • When cell receives a signal for apoptosis, one consequence is the permeability of the outer mitochondrial membrane will increase, allowing cytochrome c release. • The release of cytochrome c will activate caspase 9, which will initiate the protein ...
1 - SchoolNotes
... 79. Carbon dioxide movement into a plant leaf involves what? 80. Protons accumulate in the thylakoid space during ETC between photosystems I and II. The excess of protons are used for what? 81. What are stroma? ...
... 79. Carbon dioxide movement into a plant leaf involves what? 80. Protons accumulate in the thylakoid space during ETC between photosystems I and II. The excess of protons are used for what? 81. What are stroma? ...
Principles of Metabolic Regulation
... • Regulation of catalysis typically involves – Binding of inhibitors, often to the active site – Binding of regulatory protein subunits ...
... • Regulation of catalysis typically involves – Binding of inhibitors, often to the active site – Binding of regulatory protein subunits ...
Chapter 19a Oxidative Phosphorylation and
... During electron transfer through the mitochondrial respiratory chain, the overall reaction is: NADH + 1/2 O2 + H+ → NAD+ + H2O. The difference in reduction potentials for the two half-reactions (deltaE'°) is +1.14 V. Show how you would calculate the standard free-energy change, deltaG'°, for the rea ...
... During electron transfer through the mitochondrial respiratory chain, the overall reaction is: NADH + 1/2 O2 + H+ → NAD+ + H2O. The difference in reduction potentials for the two half-reactions (deltaE'°) is +1.14 V. Show how you would calculate the standard free-energy change, deltaG'°, for the rea ...
respiration
... • Net Reaction Appears as the Reverse of PS • The individual reactions that occur to achieve the net effect are entirely different ...
... • Net Reaction Appears as the Reverse of PS • The individual reactions that occur to achieve the net effect are entirely different ...
PowerPoint 프레젠테이션
... • Four electrons are funneled into O2 to completely reduce it to H2O and concomitantly pump protons from the matrix to the cytosolic side of the inner mitochondrial membrane. • Heme a3 and Cu from the active center at which O2 is reduced to H2O. • Cytochrome c oxidase evolved to pump 4 additional H ...
... • Four electrons are funneled into O2 to completely reduce it to H2O and concomitantly pump protons from the matrix to the cytosolic side of the inner mitochondrial membrane. • Heme a3 and Cu from the active center at which O2 is reduced to H2O. • Cytochrome c oxidase evolved to pump 4 additional H ...
RESPIRATION
... • (b) Sucrose:- It is the principal soluble disaccharide which is converted into the glucose and fructose by the action of enzyme invertase. • (C) Glucose:- A monosaccharide hexose molecule which act as chief respiratory substrate. • (d)Fructose:- It is directly converted into fructose-6-phosphate ...
... • (b) Sucrose:- It is the principal soluble disaccharide which is converted into the glucose and fructose by the action of enzyme invertase. • (C) Glucose:- A monosaccharide hexose molecule which act as chief respiratory substrate. • (d)Fructose:- It is directly converted into fructose-6-phosphate ...
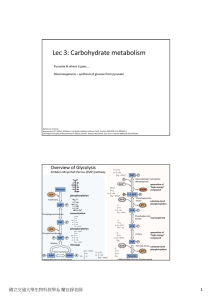
Lec 3: Carbohydrate metabolism
... • Ethanol production from pyruvate requires two enzymes: Pyruvate decarboxylase & alcohol dehydrogenase. Most common alcohol producing microbes are Saccharomyces serevisiae (baker’s yeast) and Zymomonas mobilis (bacteria) • Humans have alcohol dehydrogenase, primarily for metabolizing ethanol. Ethan ...
... • Ethanol production from pyruvate requires two enzymes: Pyruvate decarboxylase & alcohol dehydrogenase. Most common alcohol producing microbes are Saccharomyces serevisiae (baker’s yeast) and Zymomonas mobilis (bacteria) • Humans have alcohol dehydrogenase, primarily for metabolizing ethanol. Ethan ...
PPT - gserianne.com
... • Proteins with short life-spans, that are misfolded, or that become oxidized must be destroyed and recycled by the cell. Enzymes that degrade proteins are called proteases. They are hydrolytic enzymes. Most large cytosolic proteins in eukaryotes are degraded by enzyme complexes called proteasomes. ...
... • Proteins with short life-spans, that are misfolded, or that become oxidized must be destroyed and recycled by the cell. Enzymes that degrade proteins are called proteases. They are hydrolytic enzymes. Most large cytosolic proteins in eukaryotes are degraded by enzyme complexes called proteasomes. ...
Nucleotides: Be able to differentiate between a purine ring and a
... reaction. The enzyme can use the R groups of the amino acids in its chain, but sometimes something more is sometimes needed. The cofactor is found at the active site of the enzyme and participates in the catalytic mechanism. A cofactor can be: 1) a metal 2) an organic molecule – we will call these c ...
... reaction. The enzyme can use the R groups of the amino acids in its chain, but sometimes something more is sometimes needed. The cofactor is found at the active site of the enzyme and participates in the catalytic mechanism. A cofactor can be: 1) a metal 2) an organic molecule – we will call these c ...
Metabolism
... reactions that provide energy for the production of ATP • This energy is used to generate ATP from phosphorylation of ADP. • It is a series of Redox reactions. ...
... reactions that provide energy for the production of ATP • This energy is used to generate ATP from phosphorylation of ADP. • It is a series of Redox reactions. ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.