File
... Glycolysis NADH • The NADH generated during glycolysis is used to fuel mitochondrial ATP synthesis via oxidative phosphorylation, producing either two or three equivalents of ATP • Depending upon whether the glycerol phosphate shuttle or the malate-aspartate shuttle is used to transport the electro ...
... Glycolysis NADH • The NADH generated during glycolysis is used to fuel mitochondrial ATP synthesis via oxidative phosphorylation, producing either two or three equivalents of ATP • Depending upon whether the glycerol phosphate shuttle or the malate-aspartate shuttle is used to transport the electro ...
C383 Study Guide for the Final Exam Spring 2017 Basic Information
... D. Based on these data, how would you expect this inhibitor to effect pyruvate carboxylase? How would this effect gluconeogenesis? 2. Recognize names and/or chemical structures of: ATP, all amino acids, all glycolysis intermediates, acetyl CoA, all citric acid cycle intermediates. Know 1 and 3-lett ...
... D. Based on these data, how would you expect this inhibitor to effect pyruvate carboxylase? How would this effect gluconeogenesis? 2. Recognize names and/or chemical structures of: ATP, all amino acids, all glycolysis intermediates, acetyl CoA, all citric acid cycle intermediates. Know 1 and 3-lett ...
ch8and9notes2011
... and passed on to electron transport chain.new electrons come from the water,and O2 is released into air 2. High –energy electrons move from electron transport chain from photosystem II to photosystem I.Energy from electrons is used by molecules in electron transport chain to transport H+ ions from s ...
... and passed on to electron transport chain.new electrons come from the water,and O2 is released into air 2. High –energy electrons move from electron transport chain from photosystem II to photosystem I.Energy from electrons is used by molecules in electron transport chain to transport H+ ions from s ...
Cell Respiration Study Guide
... 4. What happens to pyruvic acid before it enters the Kreb cycle? 5. What is lost when pyruvic acid is converted to acetyl CoA? 6. What else is formed during the conversion of pyruvic acid to Acetyl CoA? How many per pyruvate? How many total? 7. From glycolysis and the conversion of pyruvic acid how ...
... 4. What happens to pyruvic acid before it enters the Kreb cycle? 5. What is lost when pyruvic acid is converted to acetyl CoA? 6. What else is formed during the conversion of pyruvic acid to Acetyl CoA? How many per pyruvate? How many total? 7. From glycolysis and the conversion of pyruvic acid how ...
+ energy
... •ATP + H2O ADP + Pi ΔGo’ = -30.5kJ/mol •ATP + H2O AMP + Pii ΔGo’ = -45.6kJ/mol triphosphate unit ...
... •ATP + H2O ADP + Pi ΔGo’ = -30.5kJ/mol •ATP + H2O AMP + Pii ΔGo’ = -45.6kJ/mol triphosphate unit ...
Bioenergetics of Exercise and Training
... Substrate level phosphorylation creates 1 ATP (indirectly through GTP; see section B.5.a. for more details on substrate level phosphorylation) ...
... Substrate level phosphorylation creates 1 ATP (indirectly through GTP; see section B.5.a. for more details on substrate level phosphorylation) ...
Aerobic/Anaerobic Respiration
... Identification of various respiratory types x (Cytochrome) Oxidase test Positive reaction identifies organisms which use aerobic respiration x Nitrate reductase test Positive reaction identifies organisms which use nitrate as terminal electron acceptor in anaerobic respiration ...
... Identification of various respiratory types x (Cytochrome) Oxidase test Positive reaction identifies organisms which use aerobic respiration x Nitrate reductase test Positive reaction identifies organisms which use nitrate as terminal electron acceptor in anaerobic respiration ...
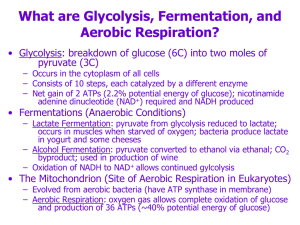
GLYCOLYSIS
... • Phosphofructokinase inhibition will cause F-6P to rise which also incr G-6P • However other sugars (such as fructose) bypass this step so it should not be only control • The inhibition of F-6P leads to inhibition in G-6P ...
... • Phosphofructokinase inhibition will cause F-6P to rise which also incr G-6P • However other sugars (such as fructose) bypass this step so it should not be only control • The inhibition of F-6P leads to inhibition in G-6P ...
Medical Biochemistry at a Glance. 3rd Edition. At a Glance Brochure
... Part 3 Formation of ATP: oxidation and reduction reactions 9 Oxidation/reduction reactions, coenzymes and prosthetic groups 26 10 Anaerobic production of ATP by substrate–level phosphorylation, from phosphocreatine and by the adenylate kinase (myokinase) reaction 28 ...
... Part 3 Formation of ATP: oxidation and reduction reactions 9 Oxidation/reduction reactions, coenzymes and prosthetic groups 26 10 Anaerobic production of ATP by substrate–level phosphorylation, from phosphocreatine and by the adenylate kinase (myokinase) reaction 28 ...
Practice Exam 2
... reacts with a fatty acid a(n) _________________________ linkage is formed along with the production of _________________________. The membranes of cells are composed of phospholipids, molecules in which one of the fatty acids has been replaced by a(n) _________________________ group which is _______ ...
... reacts with a fatty acid a(n) _________________________ linkage is formed along with the production of _________________________. The membranes of cells are composed of phospholipids, molecules in which one of the fatty acids has been replaced by a(n) _________________________ group which is _______ ...
PowerPoint 簡報
... 1. Here we focus on discussing the metabolism of glucose. For the metabolism of other organic compounds (eg. Proteins or lipids), please refer to a textbook of Biochemistry. 2. Bacteria can produce energy from glucose by fermentation (w/o O2), anaerobic reaction (w/o O2), or aerobic respiration. 3. ...
... 1. Here we focus on discussing the metabolism of glucose. For the metabolism of other organic compounds (eg. Proteins or lipids), please refer to a textbook of Biochemistry. 2. Bacteria can produce energy from glucose by fermentation (w/o O2), anaerobic reaction (w/o O2), or aerobic respiration. 3. ...
Slide 1
... • The Transition Reaction (pyruvate acetyl CoA) – Acetyl Coenzyme-A: “central character” in metabolism (can be produced from carbohydrates, lipids, and certain amino acids) – Pyruvate converted to acetyl group (2C); loss of CO2 molecule – Coenzyme-A (CoA): a large thiol derived from ATP and pantot ...
... • The Transition Reaction (pyruvate acetyl CoA) – Acetyl Coenzyme-A: “central character” in metabolism (can be produced from carbohydrates, lipids, and certain amino acids) – Pyruvate converted to acetyl group (2C); loss of CO2 molecule – Coenzyme-A (CoA): a large thiol derived from ATP and pantot ...
Chapter 14 Glycolysis Glucose 2 Pyruvate → → → 2 Lactate (sent to
... → Must have a supply of NAD+ in cytosol for glycolysis to keep going. → Under aerobic conditions, the electrons from the NADH produced in this step must be shuttled into the mitochondria using either the malate aspartate shuttle system or the α-glycerol phosphate shuttle system. The use of the shutt ...
... → Must have a supply of NAD+ in cytosol for glycolysis to keep going. → Under aerobic conditions, the electrons from the NADH produced in this step must be shuttled into the mitochondria using either the malate aspartate shuttle system or the α-glycerol phosphate shuttle system. The use of the shutt ...
skeletal ms
... It produces great amount of ATP (38 ATP). It is economic but slow. 4) In exhausted ms: There is an emergency mechanism for the supply of ATP ADP + ADP ATP + AMP ...
... It produces great amount of ATP (38 ATP). It is economic but slow. 4) In exhausted ms: There is an emergency mechanism for the supply of ATP ADP + ADP ATP + AMP ...
GOALS FOR LECTURE 7:
... All tissues in the body break down glucose to provide energy and intermediates for other metabolic and biosynthetic pathways. Virtually all sugars can be converted to glucose, so the process of glycolysis is central to carbohydrate metabolism. For cells that lack mitochondria, such as red blood cell ...
... All tissues in the body break down glucose to provide energy and intermediates for other metabolic and biosynthetic pathways. Virtually all sugars can be converted to glucose, so the process of glycolysis is central to carbohydrate metabolism. For cells that lack mitochondria, such as red blood cell ...
AnaerobicAerobic CellResp
... - Explain why you do this, making a connection to anaerobic respiration. ...
... - Explain why you do this, making a connection to anaerobic respiration. ...
Ch15 Lect F09
... group from an amino acid to an -keto acid. These reactions are catalyzed by transaminase enzymes. 2) In oxidative deamination an amino group is replaced by a carbonyl (C=O) group. ...
... group from an amino acid to an -keto acid. These reactions are catalyzed by transaminase enzymes. 2) In oxidative deamination an amino group is replaced by a carbonyl (C=O) group. ...
PPT File
... 7okg male need 8400kJ/day need 83kg ATP recycle ATP about 300 times/day Electron-transport chain (respiratory chain): electron flow NADH + ½ O2 + H+ H2O + NAD+ The proton pumps from mitochondria matrix into the intermembrane space pH gradient ...
... 7okg male need 8400kJ/day need 83kg ATP recycle ATP about 300 times/day Electron-transport chain (respiratory chain): electron flow NADH + ½ O2 + H+ H2O + NAD+ The proton pumps from mitochondria matrix into the intermembrane space pH gradient ...
Mitochondrial Energy Metabolism:
... by release of glucose from the liver (glycogen) • As we run out of glycogen we maintain the blood glucose level by making glucose from amino acids (protein) and other compounds • The energy to make glucose comes from burning fatty acids, which also generates ketones that are used as an alternative ...
... by release of glucose from the liver (glycogen) • As we run out of glycogen we maintain the blood glucose level by making glucose from amino acids (protein) and other compounds • The energy to make glucose comes from burning fatty acids, which also generates ketones that are used as an alternative ...
electron transport chain
... phosphorylation, chemiosmosis couples electron transport to ATP synthesis • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via ...
... phosphorylation, chemiosmosis couples electron transport to ATP synthesis • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via ...
BIO 101
... 26. What is the most common lipid consumed by humans? 27. Before energy can be obtained from a fat molecule, what must first happen to it? 28. What metabolic pathways are involved in the complete oxidation of a free fatty acid? ...
... 26. What is the most common lipid consumed by humans? 27. Before energy can be obtained from a fat molecule, what must first happen to it? 28. What metabolic pathways are involved in the complete oxidation of a free fatty acid? ...
Slide 1
... Lipoproteins consist of lipids and protein and are formed in the liver Blood contains three types of lipoproteins: very low density, low density, and high density Fatty acids are transported from the cells of one tissue to the cells of another in the form of free fatty acids ...
... Lipoproteins consist of lipids and protein and are formed in the liver Blood contains three types of lipoproteins: very low density, low density, and high density Fatty acids are transported from the cells of one tissue to the cells of another in the form of free fatty acids ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.