LP - Columbia University
... 53 kcal/mole released, TOO high. Too much energy released: if it were used in one fell swoop of the usual coupled reaction, we would get only a single ATP's worth, 7 kcal/mole, from this 53 kcal/mole, and we'd release a LOT of heat besides. It would be better if we could break up this -53 kcal/mole ...
... 53 kcal/mole released, TOO high. Too much energy released: if it were used in one fell swoop of the usual coupled reaction, we would get only a single ATP's worth, 7 kcal/mole, from this 53 kcal/mole, and we'd release a LOT of heat besides. It would be better if we could break up this -53 kcal/mole ...
STUDY GUIDE –Intro to Cell Biology
... Hydrogen bonds are weak interactions between the slightly positive hydrogens of one molecule and a slightly negative atoms of another molecule What are reactants? What are products? Reactants are what goes INTO the reaction (think individual ingredients when baking a cake ie. Sugar, flour, eggs). Pr ...
... Hydrogen bonds are weak interactions between the slightly positive hydrogens of one molecule and a slightly negative atoms of another molecule What are reactants? What are products? Reactants are what goes INTO the reaction (think individual ingredients when baking a cake ie. Sugar, flour, eggs). Pr ...
What is Respiration? - Deans Community High School
... The energy released from the breakdown of glucose is used to generate molecules of Adenosine triphosphate (ATP) inside cells. ATP is a chemical store of energy: it is generated from molecules of Adenosine diphosphate (ADP) and phosphate (Pi). ATP is formed when the chemical energy released from gluc ...
... The energy released from the breakdown of glucose is used to generate molecules of Adenosine triphosphate (ATP) inside cells. ATP is a chemical store of energy: it is generated from molecules of Adenosine diphosphate (ADP) and phosphate (Pi). ATP is formed when the chemical energy released from gluc ...
Growth final1 - TOP Recommended Websites
... Measuring bacterial mass (live + dead) in liquid culture ...
... Measuring bacterial mass (live + dead) in liquid culture ...
Table of Contents - Milan Area Schools
... reductase complex which in turn passes them to cytochrome c. • Next to receive them is cytochrome c oxidase complex. Then they are passed to O2. • Reduced oxygen unites with two hydrogen ions to form water. ...
... reductase complex which in turn passes them to cytochrome c. • Next to receive them is cytochrome c oxidase complex. Then they are passed to O2. • Reduced oxygen unites with two hydrogen ions to form water. ...
Reaction of glycolysis
... •1,3-bisphosphoglycerate is converted to 3 phosphoglycerate •This step (step 7) involves another reaction in which ATP is produced by phosphorylation of ADP •1,3-bisphosphoglycerate transfers a phosphate group to ADP. This is known as substrate-level phosphorylation •Reaction is catalyzed by phospho ...
... •1,3-bisphosphoglycerate is converted to 3 phosphoglycerate •This step (step 7) involves another reaction in which ATP is produced by phosphorylation of ADP •1,3-bisphosphoglycerate transfers a phosphate group to ADP. This is known as substrate-level phosphorylation •Reaction is catalyzed by phospho ...
Chapter 7 Cellular Respiration
... metabolism. The outer membrane of the mitochondria acts as a cell membrane and houses transport proteins that allow substances in and out of the mitochondria. For instance, the outer membrane houses transport proteins, which move the two pyruvate molecules formed during glycolysis from the cytoplasm ...
... metabolism. The outer membrane of the mitochondria acts as a cell membrane and houses transport proteins that allow substances in and out of the mitochondria. For instance, the outer membrane houses transport proteins, which move the two pyruvate molecules formed during glycolysis from the cytoplasm ...
TCA Cycle Handout 1
... efficiently meet the needs of the cell and the organis. The irreversible synthesis of acetyl-CoA from pyruvate by pyruvate dehydrogenase is one important regulatory step, and is inhibited by high concentrations of ATP that indicate abundant energy. Citrate synthase, alpha-ketoglutarate dehydrogenase ...
... efficiently meet the needs of the cell and the organis. The irreversible synthesis of acetyl-CoA from pyruvate by pyruvate dehydrogenase is one important regulatory step, and is inhibited by high concentrations of ATP that indicate abundant energy. Citrate synthase, alpha-ketoglutarate dehydrogenase ...
CHAPTER 4: CELLULAR METABOLISM
... Introduction: 1. CR is how animal cells use oxygen to release chemical energy from food to generate cellular energy (ATP). 2. The chemical reactions in CR must occur in a particular sequence, with each reaction being catalyzed by a different (specific) enzyme. There are three major series of reactio ...
... Introduction: 1. CR is how animal cells use oxygen to release chemical energy from food to generate cellular energy (ATP). 2. The chemical reactions in CR must occur in a particular sequence, with each reaction being catalyzed by a different (specific) enzyme. There are three major series of reactio ...
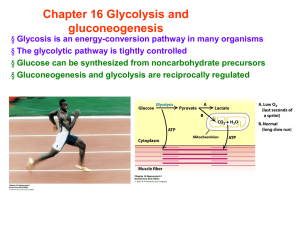
Chapter 16 Glycolysis and gluconeogenesis
... no longer requires Glc for energy or for the synthesis of glycogen Glc will be left in the blood if phosphofructokinase is inhibited [F6P] [G6P] hexokinase is inhibited ...
... no longer requires Glc for energy or for the synthesis of glycogen Glc will be left in the blood if phosphofructokinase is inhibited [F6P] [G6P] hexokinase is inhibited ...
Nucleic acids
... The two most common forms of nucleic acids are DNA and RNA. Nucleic acids are made up of smaller monomers of carbon, nitrogen, oxygen, phosphorus, and hydrogen called nucleotides. The chemical groups that make up nucleotides are phosphates, nitrogenous bases, and ribose sugars. ...
... The two most common forms of nucleic acids are DNA and RNA. Nucleic acids are made up of smaller monomers of carbon, nitrogen, oxygen, phosphorus, and hydrogen called nucleotides. The chemical groups that make up nucleotides are phosphates, nitrogenous bases, and ribose sugars. ...
Notes 3 Fermentation
... 1. The body stores energy in the form of the carbohydrate glycogen. These glycogen stores are enough to last for 15 to 20 minutes of activity. After that, the body begins to break down other stored molecules, including fats, for energy. ...
... 1. The body stores energy in the form of the carbohydrate glycogen. These glycogen stores are enough to last for 15 to 20 minutes of activity. After that, the body begins to break down other stored molecules, including fats, for energy. ...
Lipid Metabolism Catabolism Overview
... • Opposite of beta oxidation in the sense that 2‐ carbon acetate units are linked to form even‐ chain, saturated fatty acids • Differs from Fatty acid degradation – In cytoplasm, not matrix – Acyl carrier protein rather than CoA – Enzymes linked in a complex – Utilizes NADPH – Energetically linked t ...
... • Opposite of beta oxidation in the sense that 2‐ carbon acetate units are linked to form even‐ chain, saturated fatty acids • Differs from Fatty acid degradation – In cytoplasm, not matrix – Acyl carrier protein rather than CoA – Enzymes linked in a complex – Utilizes NADPH – Energetically linked t ...
Fermentation and Cellular Respiration
... The third set of chemical reactions associated with cellular respiration involves enzymes that are bound to membranes. In the case of prokaryotic cells, these membranes are cell membranes, while within most eukaryotic cells, the membranes involved are the inner folded membranes or cristae of mitocho ...
... The third set of chemical reactions associated with cellular respiration involves enzymes that are bound to membranes. In the case of prokaryotic cells, these membranes are cell membranes, while within most eukaryotic cells, the membranes involved are the inner folded membranes or cristae of mitocho ...
Energy Ch6
... • 6.3.1 ATP Is the Principal Energy Carrier in Cells – Figure 6.4 ADP and ATP (p. 104) – Unnumbered Figure 6 (Hide/Reveal) ATP synthesis: Energy is stored in ATP (p. 104) – Unnumbered Figure 7 (Hide/Reveal) ATP breakdown: Energy of ATP is released (p. 104) – Figure 6.5 Coupled reactions within livin ...
... • 6.3.1 ATP Is the Principal Energy Carrier in Cells – Figure 6.4 ADP and ATP (p. 104) – Unnumbered Figure 6 (Hide/Reveal) ATP synthesis: Energy is stored in ATP (p. 104) – Unnumbered Figure 7 (Hide/Reveal) ATP breakdown: Energy of ATP is released (p. 104) – Figure 6.5 Coupled reactions within livin ...
Chem*3560 Lecture 16: Reciprocal regulation of glycolysis and
... Although pyruvate dehydrogenase does not lie in either the glycolysis or gluconeogenesis sequences, its activity could divert pyruvate into the TCA cycle. Pyruvate dehydrogenase is regulated by product inhibition as well as by phosphorylation, which decrease activity. Product inhibition occurs when ...
... Although pyruvate dehydrogenase does not lie in either the glycolysis or gluconeogenesis sequences, its activity could divert pyruvate into the TCA cycle. Pyruvate dehydrogenase is regulated by product inhibition as well as by phosphorylation, which decrease activity. Product inhibition occurs when ...
Chapter 9 - Slothnet
... The Pathway of Electron Transport • The electron transport chain is in the inner membrane (cristae) of the mitochondrion • Most of the chain’s components are proteins, which exist in multiprotein complexes • The carriers alternate reduced and oxidized states as they accept and donate electrons • El ...
... The Pathway of Electron Transport • The electron transport chain is in the inner membrane (cristae) of the mitochondrion • Most of the chain’s components are proteins, which exist in multiprotein complexes • The carriers alternate reduced and oxidized states as they accept and donate electrons • El ...
No Slide Title - Suffolk County Community College
... - can increase reaction rates up to 10 billion X faster than random collisions allow Turnover number = maximum number of substrate molecules an enzyme converts to product each second, different for different enzymes Each enzyme has a unique 3D shape: it will bind only its specific substrate(s) at t ...
... - can increase reaction rates up to 10 billion X faster than random collisions allow Turnover number = maximum number of substrate molecules an enzyme converts to product each second, different for different enzymes Each enzyme has a unique 3D shape: it will bind only its specific substrate(s) at t ...
Chapter 8: An Introduction to Metabolism
... Just as ATP is used to power cellular processes, it is regenerated from catabolic pathways. Energy releasing processes such as cellular respiration provide energy for synthesizing ATP. ...
... Just as ATP is used to power cellular processes, it is regenerated from catabolic pathways. Energy releasing processes such as cellular respiration provide energy for synthesizing ATP. ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.