Growth final1 - TOP Recommended Websites
... – Embden Meyerhof Parnas Pathway – most bacteria – also animals and plants ...
... – Embden Meyerhof Parnas Pathway – most bacteria – also animals and plants ...
the Four Stages of Biochemical Energy Production
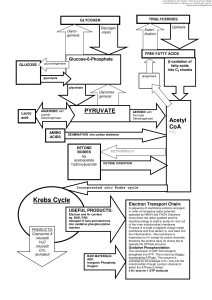
... Citric acid cycle – For every glucose, two acetyl groups enter the citric acid cycle (Krebs cycle) • Each two-carbon acetyl group combines with a fourcarbon compound • Two CO2 molecules are removed (why is this important?) • Energy captured as 1 ATP, 3 NADH, and 1 FADH2 form from each acetyl group ...
... Citric acid cycle – For every glucose, two acetyl groups enter the citric acid cycle (Krebs cycle) • Each two-carbon acetyl group combines with a fourcarbon compound • Two CO2 molecules are removed (why is this important?) • Energy captured as 1 ATP, 3 NADH, and 1 FADH2 form from each acetyl group ...
L24_Krebs
... – One of the methyl-Hs can easily come off acetyl CoA – Gives a very reactive species that reacts with oxaloacetate ...
... – One of the methyl-Hs can easily come off acetyl CoA – Gives a very reactive species that reacts with oxaloacetate ...
Introduction to metabolism
... Enzymes act by lowering the Activation Energy of a chemical reaction Refer to: Progress of Reaction Diagram Enzymes lower the activation energy by creating a stabilized intermediate state known as an “Enzyme-Substrate Complex” A typical enzymatic reaction follows steps that are similar to these: A ...
... Enzymes act by lowering the Activation Energy of a chemical reaction Refer to: Progress of Reaction Diagram Enzymes lower the activation energy by creating a stabilized intermediate state known as an “Enzyme-Substrate Complex” A typical enzymatic reaction follows steps that are similar to these: A ...
I. Background - Berks Catholic
... been completely oxidized All the H’s have been removed from glucose Electron Transport a. Reason – even though glucose has been oxidized very little energy has been released. It is all tied up in NADH. Energy must be released by passing the electrons to lower energy levels. This is done by elect ...
... been completely oxidized All the H’s have been removed from glucose Electron Transport a. Reason – even though glucose has been oxidized very little energy has been released. It is all tied up in NADH. Energy must be released by passing the electrons to lower energy levels. This is done by elect ...
Carbohydrate Catabolism in the Presence of Oxygen Releases a
... NADH oxidation is used to actively transport protons (H+) across the inner ...
... NADH oxidation is used to actively transport protons (H+) across the inner ...
Honors Biology Ch 6 Review sheet
... Honors Biology Ch 6 Review sheet 1) Compare photosynthesis and respiration. ...
... Honors Biology Ch 6 Review sheet 1) Compare photosynthesis and respiration. ...
Pyruvate and Energetics of Glycolysis
... 1. The conversion of pyruvate to ethanol also causes the ________. A) oxidation of NADH B) production of ADP C) consumption of O2 D) generation of an ion gradient across mitochondrial membranes 2. The enzyme that catalyzes the conversion of pyruvate to lactate is ________. A) lactate reductase ...
... 1. The conversion of pyruvate to ethanol also causes the ________. A) oxidation of NADH B) production of ADP C) consumption of O2 D) generation of an ion gradient across mitochondrial membranes 2. The enzyme that catalyzes the conversion of pyruvate to lactate is ________. A) lactate reductase ...
Example of the Course Test 2 10th December, 8:00, registration from
... a) reaction: CH3-CO-COOH + NAD+ + HSCoA -> CO2 + NADH + H+ + CH3-CO~SCoA describes a decarboxylation of oxaloacetate b) glucose can be metabolised to lactate in erythrocytes c) insulin activates only anabolic pathways d) adenylate kinase catalyzes this reaction: ADP + ADP = AMP + ATP 2) Choose true ...
... a) reaction: CH3-CO-COOH + NAD+ + HSCoA -> CO2 + NADH + H+ + CH3-CO~SCoA describes a decarboxylation of oxaloacetate b) glucose can be metabolised to lactate in erythrocytes c) insulin activates only anabolic pathways d) adenylate kinase catalyzes this reaction: ADP + ADP = AMP + ATP 2) Choose true ...
T/F 1. Pyruvate, the end product of glycolysis, is processed
... 2. In lactic acid fermentation pyruvate is reduced to pyruvic acid. 3. In ethanol fermentation, pyruvate is converted to acetaldehyde which is reduced to ethanol 4. During fermentation NAD+ is educed to NADH, allowing glycolysis to proceed 5. Glycolysis is an ancient biochemical pathway that was lik ...
... 2. In lactic acid fermentation pyruvate is reduced to pyruvic acid. 3. In ethanol fermentation, pyruvate is converted to acetaldehyde which is reduced to ethanol 4. During fermentation NAD+ is educed to NADH, allowing glycolysis to proceed 5. Glycolysis is an ancient biochemical pathway that was lik ...
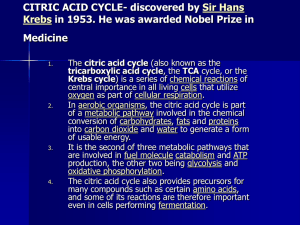
CITRIC ACID CYCLE
... It is the second of three metabolic pathways that are involved in fuel molecule catabolism and ATP production, the other two being glycolysis and oxidative phosphorylation. The citric acid cycle also provides precursors for many compounds such as certain amino acids, and some of its reactions are th ...
... It is the second of three metabolic pathways that are involved in fuel molecule catabolism and ATP production, the other two being glycolysis and oxidative phosphorylation. The citric acid cycle also provides precursors for many compounds such as certain amino acids, and some of its reactions are th ...
Make It – Break It
... From the indicated starting compound(s) use metabolic pathways to make one molecule of the indicated compound. For this assignment portion, you can assume that you have all ATP or NAD(P)H and non-organic substrates (e.g. ammonia) needed for biosynthetic reactions. Diagram the pathways involved, clea ...
... From the indicated starting compound(s) use metabolic pathways to make one molecule of the indicated compound. For this assignment portion, you can assume that you have all ATP or NAD(P)H and non-organic substrates (e.g. ammonia) needed for biosynthetic reactions. Diagram the pathways involved, clea ...
KEY - chem.uwec.edu
... Assuming the oysters have a steady supply of oxaloacetate (from amino acids), how much energy could they derive from this process (per “cycle”)? One ATP “equivalent” is generated by succinyl CoA synthetase. The NADH used cancels the NADH produced and the second NADH can reduce FAD via the electron t ...
... Assuming the oysters have a steady supply of oxaloacetate (from amino acids), how much energy could they derive from this process (per “cycle”)? One ATP “equivalent” is generated by succinyl CoA synthetase. The NADH used cancels the NADH produced and the second NADH can reduce FAD via the electron t ...
Nuclear Chemistry
... There is a net formation of _______ mol of ATP and _______ mol of physiological reductant NADH per mole of ______________ consumed. ...
... There is a net formation of _______ mol of ATP and _______ mol of physiological reductant NADH per mole of ______________ consumed. ...
BIOL 101 Cellular Respiration I. Organic Molecules A. Energy input
... 1. strip away electrons from chemical bonds 2. oxidation of food molecules - cellular respiration - 2 step process (remove e- then use) II. Glycolysis (first step) - in cytoplasm A. Splitting of glucose 1. 9 enzyme-catalyzed reactions 2. glucose → two 3-C molecules 3. pyruvate B. Substrate-level pho ...
... 1. strip away electrons from chemical bonds 2. oxidation of food molecules - cellular respiration - 2 step process (remove e- then use) II. Glycolysis (first step) - in cytoplasm A. Splitting of glucose 1. 9 enzyme-catalyzed reactions 2. glucose → two 3-C molecules 3. pyruvate B. Substrate-level pho ...
UNIT-1 Carbohydrates
... Function: quick energy structural support Characteristics: H – C – OH ratio of hydrogen to oxygen atoms is 2:1 Monomer is the monosaccharide ...
... Function: quick energy structural support Characteristics: H – C – OH ratio of hydrogen to oxygen atoms is 2:1 Monomer is the monosaccharide ...
Cellular Respiration
... Lactic Acid Fermentation • The enzyme lactate dehydrogenase converts pyruvate into lactic acid and converts NADH into NAD+. • Usually blood can remove the lactate, however if this does not happen muscle fatigue results. ...
... Lactic Acid Fermentation • The enzyme lactate dehydrogenase converts pyruvate into lactic acid and converts NADH into NAD+. • Usually blood can remove the lactate, however if this does not happen muscle fatigue results. ...
Ch16
... CoA-SH - becomes the thioester. FAD – oxidizes reduced lipoic acid. NAD+ - oxidizes FADH2 to FAD becoming NADH +H+. ...
... CoA-SH - becomes the thioester. FAD – oxidizes reduced lipoic acid. NAD+ - oxidizes FADH2 to FAD becoming NADH +H+. ...
Ch 9 Power Point - Cellular Respiration
... • NAD+ (nicotinamide adenine dinucleotide) – coenzyme – eacceptor • Removes electrons from food (series of reactions) • NAD+ is reduced to NADH • Enzyme action: dehydrogenase – Removes a pair of H atoms from the substrate (sugar) & delivers 2e- and 1 proton (H+) to NAD+ - releases other H+ • Oxygen ...
... • NAD+ (nicotinamide adenine dinucleotide) – coenzyme – eacceptor • Removes electrons from food (series of reactions) • NAD+ is reduced to NADH • Enzyme action: dehydrogenase – Removes a pair of H atoms from the substrate (sugar) & delivers 2e- and 1 proton (H+) to NAD+ - releases other H+ • Oxygen ...
Principles of Energy Harvest Redox reactions Oxidizing agent in
... • Electron Transport Chain: inner membrane of mitochondrion; electrons passed to oxygen ...
... • Electron Transport Chain: inner membrane of mitochondrion; electrons passed to oxygen ...
Chapter 9. Cellular Respiration STAGE 1: Glycolysis
... 2. Some organisms that are exposed to oxygen, but switch to fermentation when oxygen is scarce. AP Biology ...
... 2. Some organisms that are exposed to oxygen, but switch to fermentation when oxygen is scarce. AP Biology ...
1 - BrainMass
... a. Draw the basic structure of each (two glucose units in the main chain and one in the branch is sufficient), numbering all atoms b. Compare and contrast the function of these related compounds c. What is/are the molecular reasons for this functional difference? d. How do you suppose a cell can mak ...
... a. Draw the basic structure of each (two glucose units in the main chain and one in the branch is sufficient), numbering all atoms b. Compare and contrast the function of these related compounds c. What is/are the molecular reasons for this functional difference? d. How do you suppose a cell can mak ...
Fermentation 2015: The ABE process
... producers of industrial chemicals. Despite this, the science behind most renewable chemicals is often poorly understood, due to many processes being protected by the companies and institutions behind the technologies. Fermentation science, however, is an ancient art and most of the details of how fe ...
... producers of industrial chemicals. Despite this, the science behind most renewable chemicals is often poorly understood, due to many processes being protected by the companies and institutions behind the technologies. Fermentation science, however, is an ancient art and most of the details of how fe ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.