LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 3. Write the components that are involved in the synthesis of acetyl coenzyme A. 4. What would be the decarboxylated product of pyruvate in glycolysis? Mention the structure. 5. Define glycosuria. 6. What are ketone bodies? When and how are they formed in the body? 7. Calculate the energitics for pa ...
... 3. Write the components that are involved in the synthesis of acetyl coenzyme A. 4. What would be the decarboxylated product of pyruvate in glycolysis? Mention the structure. 5. Define glycosuria. 6. What are ketone bodies? When and how are they formed in the body? 7. Calculate the energitics for pa ...
File - Mr. Shanks` Class
... _____________________ molecules. a) 2 glyceraldehyde – 3 - phosphate b) 3 pyruvate c) 2 phosphoenol pyruvate d) 2 pyruvate 12. The transformation of fumarate into malate requires the use of one enzyme: a) Dehydrogenase b) Phosphorylase c) Isomerase d) Hydrase 13. Approximately how much energy is in ...
... _____________________ molecules. a) 2 glyceraldehyde – 3 - phosphate b) 3 pyruvate c) 2 phosphoenol pyruvate d) 2 pyruvate 12. The transformation of fumarate into malate requires the use of one enzyme: a) Dehydrogenase b) Phosphorylase c) Isomerase d) Hydrase 13. Approximately how much energy is in ...
A2 Aerobic respiration Link reaction Glucose cannot cross the
... _____________ or the formation of the proton gradient they stop ATP synthesis. In this situation electrons cannot be passed to ___________, so it is not used up. Also as energy cannot be passed to generate ATP it is lost as _______, warming the surroundings further. In the presence of inhibitors, ce ...
... _____________ or the formation of the proton gradient they stop ATP synthesis. In this situation electrons cannot be passed to ___________, so it is not used up. Also as energy cannot be passed to generate ATP it is lost as _______, warming the surroundings further. In the presence of inhibitors, ce ...
скачати - ua
... Under anaerobic conditions, the absence of oxygen, pyruvic acid can be routed by the organism into one of three pathways: lactic acid fermentation, alcohol fermentation, or cellular (anaerobic) respiration. Humans cannot ferment alcohol in their own bodies, we lack the genetic information to do so. ...
... Under anaerobic conditions, the absence of oxygen, pyruvic acid can be routed by the organism into one of three pathways: lactic acid fermentation, alcohol fermentation, or cellular (anaerobic) respiration. Humans cannot ferment alcohol in their own bodies, we lack the genetic information to do so. ...
Biomaterial-Nanoparticle Hybrid Systems for
... based on biomolecule-NPs hybrid systems were developed. The elctrical contacting of redoxenzymes, e.g. glucose oxidase, was accomplished by the reconstitution of the apo-enzyme on a flavin adenine dinucleotide (FAD)-functionalized Au-NP (1.2 nm). The enzyme reconstituted with the Au-NP was assembled ...
... based on biomolecule-NPs hybrid systems were developed. The elctrical contacting of redoxenzymes, e.g. glucose oxidase, was accomplished by the reconstitution of the apo-enzyme on a flavin adenine dinucleotide (FAD)-functionalized Au-NP (1.2 nm). The enzyme reconstituted with the Au-NP was assembled ...
Cellular Respiration
... - does not require O2 ; occurs in cytoplasm Pyruvate Oxidation: chemical pathway that connects glycolysis to Krebs cycle 2 pyruvate molecules are moved from the cytoplasm to the matrix of the mitochondria CO2 is removed from each pyruvate molecule and released as a waste product (1/3 of what y ...
... - does not require O2 ; occurs in cytoplasm Pyruvate Oxidation: chemical pathway that connects glycolysis to Krebs cycle 2 pyruvate molecules are moved from the cytoplasm to the matrix of the mitochondria CO2 is removed from each pyruvate molecule and released as a waste product (1/3 of what y ...
Metabolism of fats and proteins
... Is oxygen required for the electron transport chain to function? If so, what is its role? The electron transport chain is where oxidative phosphorylation occurs. Where does the oxidation occur? How about the phosphorylation? ...
... Is oxygen required for the electron transport chain to function? If so, what is its role? The electron transport chain is where oxidative phosphorylation occurs. Where does the oxidation occur? How about the phosphorylation? ...
CELLULAR RESPIRATION
... The citric acid enters the Krebs cycle and is converted into carbon dioxide (a waste product), ATP, NADH, and FADH2 The NADH and FADH2 can now enter the electron transport chain These reactions take place in the mitochondria ...
... The citric acid enters the Krebs cycle and is converted into carbon dioxide (a waste product), ATP, NADH, and FADH2 The NADH and FADH2 can now enter the electron transport chain These reactions take place in the mitochondria ...
CELLULAR RESPIRATION
... The citric acid enters the Krebs cycle and is converted into carbon dioxide (a waste product), ATP, NADH, and FADH2 The NADH and FADH2 can now enter the electron transport chain These reactions take place in the mitochondria ...
... The citric acid enters the Krebs cycle and is converted into carbon dioxide (a waste product), ATP, NADH, and FADH2 The NADH and FADH2 can now enter the electron transport chain These reactions take place in the mitochondria ...
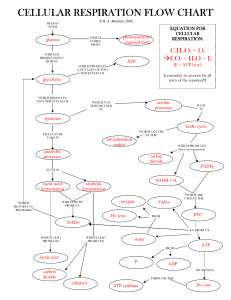
cellular respiration flow chart cellular respiration flow
... CELLULAR RESPIRATION FLOW CHART © R. A. Mathiak 2009 ...
... CELLULAR RESPIRATION FLOW CHART © R. A. Mathiak 2009 ...
BIOS 1700 Dr. Tanda Week 6, Session 3 1. What two subunits made
... ATP synthase less effective. In other words, the F0 subunit let protons go through without efficiently turning its “fan.” This means the conversion of potential energy in the proton gradient across the inner membrane to kinetic energy is less efficient. How does this mutant mouse look like compared ...
... ATP synthase less effective. In other words, the F0 subunit let protons go through without efficiently turning its “fan.” This means the conversion of potential energy in the proton gradient across the inner membrane to kinetic energy is less efficient. How does this mutant mouse look like compared ...
Respiration, Chapter 8
... • Diffusion of protons (H+) through channels (down their proton gradient) provides energy to synthesize ATP ...
... • Diffusion of protons (H+) through channels (down their proton gradient) provides energy to synthesize ATP ...
fates of pyruvate
... 2)lactic acid fermentation - pyruvate converted to lactic acid (cheese, yogurt) - Aerobic conditions: Pyruvate enter the mitochondria where it is completely oxidized Pyruvate -> enzyme -> acetyl group + CO2 + NADH ...
... 2)lactic acid fermentation - pyruvate converted to lactic acid (cheese, yogurt) - Aerobic conditions: Pyruvate enter the mitochondria where it is completely oxidized Pyruvate -> enzyme -> acetyl group + CO2 + NADH ...
Cell Respiration
... Due to the small volume of this space, it quickly becomes concentrated with protons. This creates 2 areas with different proton concentrations – LOW in matrix, HIGH in intermembrane space. ...
... Due to the small volume of this space, it quickly becomes concentrated with protons. This creates 2 areas with different proton concentrations – LOW in matrix, HIGH in intermembrane space. ...
This is Most of an Old Exam
... Cellular oxidation of food fuels is the immediate source of electrons for oxidative phosphorylation. B. In oxidative phosphorylation, both the electron transport proteins and the ATP synthase molecules are in the same membrane. C. NAD+ and FAD+ are hydrogen carrier molecules. NAD+ can carry one hydr ...
... Cellular oxidation of food fuels is the immediate source of electrons for oxidative phosphorylation. B. In oxidative phosphorylation, both the electron transport proteins and the ATP synthase molecules are in the same membrane. C. NAD+ and FAD+ are hydrogen carrier molecules. NAD+ can carry one hydr ...
Note sheet Chap 5, Sect 3
... Electrons donated by __NADH__ and __FADH2__ pass to an electron transport chain in the _folds__ of the __mitochondrion_____. ___H+ ions___ are pumped across the membrane: forming __H2O__ when combine with oxygen ; and help to produce __ATP____. A total of __up to 34____ ATP are produced for each mol ...
... Electrons donated by __NADH__ and __FADH2__ pass to an electron transport chain in the _folds__ of the __mitochondrion_____. ___H+ ions___ are pumped across the membrane: forming __H2O__ when combine with oxygen ; and help to produce __ATP____. A total of __up to 34____ ATP are produced for each mol ...
• Microbial Metabolism • What is metabolism? • All chemical
... In biological systems, the electrons are often associated with hydrogen atoms. Biological oxidations are often dehydrogenations. What happens in carbohydrate catabolism? Glucose usually is substrate ...
... In biological systems, the electrons are often associated with hydrogen atoms. Biological oxidations are often dehydrogenations. What happens in carbohydrate catabolism? Glucose usually is substrate ...
Camp 1 - Evangel University
... • In ________, large molecules are broken down to smaller products, releasing energy and transferring electrons to acceptor molecules of various sorts. The overall process is one of oxidation. • In ________, small molecules react to give rise to larger ones; this process requires energy and involves ...
... • In ________, large molecules are broken down to smaller products, releasing energy and transferring electrons to acceptor molecules of various sorts. The overall process is one of oxidation. • In ________, small molecules react to give rise to larger ones; this process requires energy and involves ...
Chapter 13 - TCA Cycle
... The outer membrane is leaky and lets pyruvate from glycolysis pass through. The inner membrane contains a transporter to move pyruvate into the matrix. ...
... The outer membrane is leaky and lets pyruvate from glycolysis pass through. The inner membrane contains a transporter to move pyruvate into the matrix. ...
Chapter 1 Homework - due Tuesday, Sept
... the operation of a) glycolysis, b) the formation of acetyl CoA, c) the citric acid cycle, and d) the electron transport chain and chemiosmosis? Please note whether the ATPs (if any) produced are made via substrate-level phosphorylation or oxidative phosphorylation. a) b) c) ...
... the operation of a) glycolysis, b) the formation of acetyl CoA, c) the citric acid cycle, and d) the electron transport chain and chemiosmosis? Please note whether the ATPs (if any) produced are made via substrate-level phosphorylation or oxidative phosphorylation. a) b) c) ...
Distinguish between - mvhs
... Anaerobic Process: Process that does not require oxygen to occur. ...
... Anaerobic Process: Process that does not require oxygen to occur. ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.