Document
... 9. The only usable ATP is produced here in the TCA cycle? a. Between Malate and Fumerate b. Between Succinate and Fumerate c. Between Succinyl Co-A and Succinate d. Between Succinate and Fumerate ______________________________________________________________________________________________ _________ ...
... 9. The only usable ATP is produced here in the TCA cycle? a. Between Malate and Fumerate b. Between Succinate and Fumerate c. Between Succinyl Co-A and Succinate d. Between Succinate and Fumerate ______________________________________________________________________________________________ _________ ...
Module 10: Catabolism of Amino Acids
... b. Why does the overall glycolysis pathway yields two and not one molecule of NADH? Breaking down glycogen yields glucose-6-phosphate without spending one molecule of ATP. How many molecules of ATP will yield each molecule of glucose coming from glycogen when its converted into pyruvate? In some bac ...
... b. Why does the overall glycolysis pathway yields two and not one molecule of NADH? Breaking down glycogen yields glucose-6-phosphate without spending one molecule of ATP. How many molecules of ATP will yield each molecule of glucose coming from glycogen when its converted into pyruvate? In some bac ...
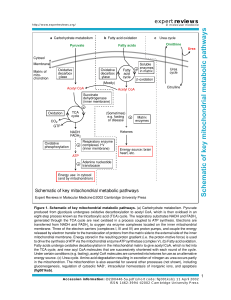
Schematic of key mitochondrial metabolic pathways
... transferred from NADH and FADH2 to oxygen via enzyme complexes located on the inner mitochondrial membrane. Three of the electron carriers (complexes I, III and IV) are proton pumps, and couple the energy released by electron transfer to the translocation of protons from the matrix side to the exter ...
... transferred from NADH and FADH2 to oxygen via enzyme complexes located on the inner mitochondrial membrane. Three of the electron carriers (complexes I, III and IV) are proton pumps, and couple the energy released by electron transfer to the translocation of protons from the matrix side to the exter ...
AP Respiration Test Review
... 2. What is the sum total of all chemical reactions within an organism? 3. What is the term for the metabolic pathways that release stored energy by breaking down complex molecules? 4. What is the term for the metabolic pathways that use store energy to build macromoleulces? 5. What is the primary ro ...
... 2. What is the sum total of all chemical reactions within an organism? 3. What is the term for the metabolic pathways that release stored energy by breaking down complex molecules? 4. What is the term for the metabolic pathways that use store energy to build macromoleulces? 5. What is the primary ro ...
Check Your Knowledge QuestionSet 2(Download)
... Q.7-A 42-year-old obese female presented to theemergency center with complaints of worsening nausea, vomiting, and abdominal pain.Her pain was located in the midepigastric area and right upper quadrant. Blood biochemistry revealed high serum amylase level.What is the probable diagnosis for this pati ...
... Q.7-A 42-year-old obese female presented to theemergency center with complaints of worsening nausea, vomiting, and abdominal pain.Her pain was located in the midepigastric area and right upper quadrant. Blood biochemistry revealed high serum amylase level.What is the probable diagnosis for this pati ...
Reading GuideChapter6_Tues
... the same site as the substrate. If the active site is occupied, then the substrate can not be turned into product….and enzyme activity is decreased. A good example of a competitive inhibitor is the drug sulfanilamide. This drug is chemically similar enough to the compound PABA. PABA is a precursor u ...
... the same site as the substrate. If the active site is occupied, then the substrate can not be turned into product….and enzyme activity is decreased. A good example of a competitive inhibitor is the drug sulfanilamide. This drug is chemically similar enough to the compound PABA. PABA is a precursor u ...
生物化學小考(一) 範圍ch1~ch4
... 4. Which of the following statements about starch and glycogen is false? (A) Amylose is unbranched; amylopectin and glycogen contain many (α-1,6) branches. (B) Both are homopolymers of glucose. (C) Both serve primarily as structural elements in cell walls. (D) Both starch and glycogen are stored int ...
... 4. Which of the following statements about starch and glycogen is false? (A) Amylose is unbranched; amylopectin and glycogen contain many (α-1,6) branches. (B) Both are homopolymers of glucose. (C) Both serve primarily as structural elements in cell walls. (D) Both starch and glycogen are stored int ...
Respiration - Biology Innovation
... The Krebs cycle happens twice as much as glycolysis because at the end of glycolysis two, three carbon pyruvates are formed and only one is used during each turn of the krebs cycle. ...
... The Krebs cycle happens twice as much as glycolysis because at the end of glycolysis two, three carbon pyruvates are formed and only one is used during each turn of the krebs cycle. ...
Exam 2 Practice #3
... Proteins, allosteric regulators c. Polynucleotides, energy carriers d. Polynucleotides, allosteric regulators ...
... Proteins, allosteric regulators c. Polynucleotides, energy carriers d. Polynucleotides, allosteric regulators ...
I. Metabolism
... ATP (adenosine triphosphate) that possesses high-energy phosphoanhydride bonds. ...
... ATP (adenosine triphosphate) that possesses high-energy phosphoanhydride bonds. ...
File
... carrier (NADH dehydrogenase) breaks NADH up into NAD+, H+ and 2 electrons. Energy is released as the two electrons get taken up by the carrier and passed on down the ...
... carrier (NADH dehydrogenase) breaks NADH up into NAD+, H+ and 2 electrons. Energy is released as the two electrons get taken up by the carrier and passed on down the ...
chapter-23
... 25. There are many biological molecules that contain high-energy phosphate bonds. ATP is used to power life processes because the energy of hydrolysis of ATP is ________. a. intermediate between the energies of hydrolysis of other organophosphate molecules b. small enough that ADP can easily be recy ...
... 25. There are many biological molecules that contain high-energy phosphate bonds. ATP is used to power life processes because the energy of hydrolysis of ATP is ________. a. intermediate between the energies of hydrolysis of other organophosphate molecules b. small enough that ADP can easily be recy ...
Bio102 Problems
... lipid must be more oxidized more times than each carbon atom from a carbohydrate. With each of these oxidations, a reduced coenzyme is produced which will ultimately be used to synthesize more ATP by oxidative phosphorylation. 16. The AMPK enzyme becomes active when A. PFK activity is inhibited. B. ...
... lipid must be more oxidized more times than each carbon atom from a carbohydrate. With each of these oxidations, a reduced coenzyme is produced which will ultimately be used to synthesize more ATP by oxidative phosphorylation. 16. The AMPK enzyme becomes active when A. PFK activity is inhibited. B. ...
DiscBio: C9 Voc Definitions
... 3. disclike clusters of pigment complexed with proteins in the thylakoids 4. adenosine triphosphate; energy molecule of the cell 5. enzyme found in the thylakoid membrane responsible for synthesis of ATP 6. series of enzyme-assisted chemical reactions producing a 3-C compound using CO2 & the energy ...
... 3. disclike clusters of pigment complexed with proteins in the thylakoids 4. adenosine triphosphate; energy molecule of the cell 5. enzyme found in the thylakoid membrane responsible for synthesis of ATP 6. series of enzyme-assisted chemical reactions producing a 3-C compound using CO2 & the energy ...
Sample Exam 2 Questions
... 8. How many ATP molecules are synthesized directly in the Krebs cycle if you supply aerobically respiring cells with 10 pyruvate molecules? A. 2 B. 5 C. 10 D. 20 E. 300 9. In cellular metabolism, O2 is used A. to provide electrons for photophosphoryation. B. in glycolysis. C. in fermentation. D. as ...
... 8. How many ATP molecules are synthesized directly in the Krebs cycle if you supply aerobically respiring cells with 10 pyruvate molecules? A. 2 B. 5 C. 10 D. 20 E. 300 9. In cellular metabolism, O2 is used A. to provide electrons for photophosphoryation. B. in glycolysis. C. in fermentation. D. as ...
Chapter 1 Homework - due Tuesday, Sept
... the operation of a) glycolysis, b) the formation of acetyl CoA, c) the citric acid cycle, and d) the electron transport chain and chemiosmosis? Please note whether the ATPs (if any) produced are made via substrate-level phosphorylation or oxidative phosphorylation. a) b) c) ...
... the operation of a) glycolysis, b) the formation of acetyl CoA, c) the citric acid cycle, and d) the electron transport chain and chemiosmosis? Please note whether the ATPs (if any) produced are made via substrate-level phosphorylation or oxidative phosphorylation. a) b) c) ...
9.2 The Process of Respiration
... membrane by NADH and FADH are dropped off at the beginning (Cytochrome A) 2. As the electrons are passed along, their energy is used to pump H+ ions out of the matrix and into the intermembrane space ...
... membrane by NADH and FADH are dropped off at the beginning (Cytochrome A) 2. As the electrons are passed along, their energy is used to pump H+ ions out of the matrix and into the intermembrane space ...
Chapter 16
... 11. Hydrolysis of the “high energy” S~C bond of succinyl-CoA produces a “high energy” GTP from GDP. GTP is converted to ATP by nucleoside diphosphate kinase. 12. Malonate inhibits succinate dehydrogenase since it is structural analog of succinate. 13. The oxidation of alkane to alkine is sufficient ...
... 11. Hydrolysis of the “high energy” S~C bond of succinyl-CoA produces a “high energy” GTP from GDP. GTP is converted to ATP by nucleoside diphosphate kinase. 12. Malonate inhibits succinate dehydrogenase since it is structural analog of succinate. 13. The oxidation of alkane to alkine is sufficient ...
energy carrier!
... ETS (cytochrome chain) is a series of reduction/oxidation reactions Enzymes embedded in mitochondrial membranes ...
... ETS (cytochrome chain) is a series of reduction/oxidation reactions Enzymes embedded in mitochondrial membranes ...
Oxidation-Reduction Enzymes
... A major part of energy employed by organisms originates from oxidation-reduction (redox) processes. Oxidized products of a redox reaction contain less potential energy (free enthalpy or Gibbs energy G) than the original reacting substances, and the difference in energy content ∆G may appear as heat ...
... A major part of energy employed by organisms originates from oxidation-reduction (redox) processes. Oxidized products of a redox reaction contain less potential energy (free enthalpy or Gibbs energy G) than the original reacting substances, and the difference in energy content ∆G may appear as heat ...
Citrate cycle - 3.LF UK 2015
... The substances enter the CC a) acetyl~CoA (→ 2 CO2) b) NAD+ and FAD (→ NADH+H+ + FADH2) c) carbon skeleton of amino acids d) GDP (→ GTP) ...
... The substances enter the CC a) acetyl~CoA (→ 2 CO2) b) NAD+ and FAD (→ NADH+H+ + FADH2) c) carbon skeleton of amino acids d) GDP (→ GTP) ...
Exam #1 Graduate: PEP 426 Intermediate Exercise Physiology
... 3. NAD+ has the greatest reduction potential of all electron transport molecules in the mitochondria. 4. AMP is an allosteric activator of both phosphorylase and phosphofructokinase. 5. Palmitate is the main fatty acid metabolized in the body. 6. Glycogenolysis needs ATP for phosphate addition to gl ...
... 3. NAD+ has the greatest reduction potential of all electron transport molecules in the mitochondria. 4. AMP is an allosteric activator of both phosphorylase and phosphofructokinase. 5. Palmitate is the main fatty acid metabolized in the body. 6. Glycogenolysis needs ATP for phosphate addition to gl ...
Chem464 Abrol Spring2017 FlippedReview4
... experiment consists of incubating a small amount of 14C-labeled substrate (the pulse) with the yeast extract just long enough for each intermediate in the fermentation pathway to become labeled. The label is then “chased” through the pathway by the addition of excess unlabeled glucose. The chase eff ...
... experiment consists of incubating a small amount of 14C-labeled substrate (the pulse) with the yeast extract just long enough for each intermediate in the fermentation pathway to become labeled. The label is then “chased” through the pathway by the addition of excess unlabeled glucose. The chase eff ...
A1988N971500002
... residue in AlP. Thus primed with the omnipresence and general importance of activated groups in biochemical processes, it seemed only natural that my interest turned to nicotinamide adenine dinucleotide (NAD) as a form of activated ADP-ribose. Use of this pyridine nucleotide as a substrate of ADP-ri ...
... residue in AlP. Thus primed with the omnipresence and general importance of activated groups in biochemical processes, it seemed only natural that my interest turned to nicotinamide adenine dinucleotide (NAD) as a form of activated ADP-ribose. Use of this pyridine nucleotide as a substrate of ADP-ri ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.