Microbial Metabolism
... The energy from the transfer of electrons along thechain transports protons across the membrane and creates an electrochemical gradient. As the accumulating protons follow the electrochemical gradient back across the membrane through an ATP synthase complex, the movement of the protons provides ener ...
... The energy from the transfer of electrons along thechain transports protons across the membrane and creates an electrochemical gradient. As the accumulating protons follow the electrochemical gradient back across the membrane through an ATP synthase complex, the movement of the protons provides ener ...
Cellular Respiration
... • NADH passes electrons to an electron transport chain • As electrons “fall” from carrier to carrier and finally to O2 • Energy is released in small quantities NAD+ NADH ...
... • NADH passes electrons to an electron transport chain • As electrons “fall” from carrier to carrier and finally to O2 • Energy is released in small quantities NAD+ NADH ...
Characterization of NAD Salvage Pathways and their Role in
... Rodionov et al., 2008; Sorci et al., 2013; Spector et al., 1985; Zhu et al., 1988). While the de ...
... Rodionov et al., 2008; Sorci et al., 2013; Spector et al., 1985; Zhu et al., 1988). While the de ...
2.2 cellular respiration: the details
... glycolysis, pyruvate oxidation, and the Krebs cycle and carry it to power ATP synthesis by oxidative phosphorylation. NAD+ is used to shuttle electrons to the first component of the electron transport chain. During oxidative phosphorylation, NAD+ removes two hydrogen atoms from a part of the origina ...
... glycolysis, pyruvate oxidation, and the Krebs cycle and carry it to power ATP synthesis by oxidative phosphorylation. NAD+ is used to shuttle electrons to the first component of the electron transport chain. During oxidative phosphorylation, NAD+ removes two hydrogen atoms from a part of the origina ...
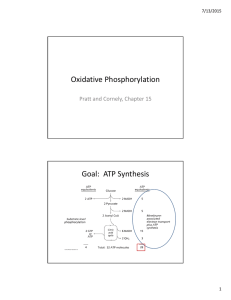
Oxidative Phosphorylation Goal: ATP Synthesis
... Glycerol‐3‐phosphate Shuttle • Glycerol phosphate shuttle (1.5 ATP/NADH) • Produces QH2 • Operational in some tissues/circumstances ...
... Glycerol‐3‐phosphate Shuttle • Glycerol phosphate shuttle (1.5 ATP/NADH) • Produces QH2 • Operational in some tissues/circumstances ...
Cell Energy: Fermentation
... then ATP will be produced using the energy of the high-energy electrons carried by NADH or FADH2 to the electron transport chain. If aerobic respiration does not occur, NADH must be reoxidized to NAD+ for reuse as an electron carrier for glycolysis to continue. How is this done? Some living systems ...
... then ATP will be produced using the energy of the high-energy electrons carried by NADH or FADH2 to the electron transport chain. If aerobic respiration does not occur, NADH must be reoxidized to NAD+ for reuse as an electron carrier for glycolysis to continue. How is this done? Some living systems ...
Assignment 5 Bioenergy/ Photosynthesis
... energy by being able to bind to the substrate and then change their conformation to force the reaction. Denaturation of a protein is a process by which some factors, like temperature or pH, affect the shape of a protein by breaking or disrupting the bonds (H-bonds, R-R interactions, or ionic interac ...
... energy by being able to bind to the substrate and then change their conformation to force the reaction. Denaturation of a protein is a process by which some factors, like temperature or pH, affect the shape of a protein by breaking or disrupting the bonds (H-bonds, R-R interactions, or ionic interac ...
lec27_2013 - Andrew.cmu.edu
... Citric acid (TCA, Krebs) cycle Electron transport Oxidative phosphorylation (ATP synthesis) ...
... Citric acid (TCA, Krebs) cycle Electron transport Oxidative phosphorylation (ATP synthesis) ...
06_Isoenzymes. Enzymodiagnostics. Enzymopathy. Enzymotherapy
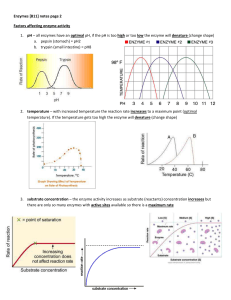
... enzyme is stable. The rate of most enzymatic reactions doubles for each 100 C rise in temperature. This is true only up to about 500 C. Above this temperature, we observe heat inactivation of enzymes. The optimum temperature of an enzyme is that temperature at which the greatest amount of substrate ...
... enzyme is stable. The rate of most enzymatic reactions doubles for each 100 C rise in temperature. This is true only up to about 500 C. Above this temperature, we observe heat inactivation of enzymes. The optimum temperature of an enzyme is that temperature at which the greatest amount of substrate ...
Nutrition & Metabolism
... Found in seafood, table salt Needed for water balance, proper nerve and muscle function ...
... Found in seafood, table salt Needed for water balance, proper nerve and muscle function ...
7.2 Glycolysis
... a) 2 ATP are used b) Oxidation – reduction reaction 2 NAD+ 2NADH + 2H+ a) 4 ATP are made b) 2 pyruvate molecules are made ...
... a) 2 ATP are used b) Oxidation – reduction reaction 2 NAD+ 2NADH + 2H+ a) 4 ATP are made b) 2 pyruvate molecules are made ...
oxidation
... three main stages Stage 3: Oxidative phosphorylation – involves electrons carried by NADH and FADH2, – shuttles these electrons to the electron transport chain embedded in the inner mitochondrial membrane, – involves chemiosmosis, and – generates ATP through oxidative phosphorylation associated wi ...
... three main stages Stage 3: Oxidative phosphorylation – involves electrons carried by NADH and FADH2, – shuttles these electrons to the electron transport chain embedded in the inner mitochondrial membrane, – involves chemiosmosis, and – generates ATP through oxidative phosphorylation associated wi ...
09 Respiration
... synthase to rotate. – The spinning rod causes a conformational change in the knob region, activating catalytic sites where ADP and inorganic Fig. 9.14 phosphate combine to ATP. Copyrightmake © 2002 Pearson Education, Inc., publishing as Benjamin Cummings ...
... synthase to rotate. – The spinning rod causes a conformational change in the knob region, activating catalytic sites where ADP and inorganic Fig. 9.14 phosphate combine to ATP. Copyrightmake © 2002 Pearson Education, Inc., publishing as Benjamin Cummings ...
BY 123 Mock Exam #2 Answer Key Chapters 8,9,10,12,13 Catabolic
... d. Water is a reducing agent e. Oxygen is a reducing agent Some prokaryotes use anaerobic respiration, a process that: a. Does not involve an electron transport chain b. Produces ATP solely by substrate-level phosphorylation c. Uses a substance other than oxygen as the final electron acceptor d. Doe ...
... d. Water is a reducing agent e. Oxygen is a reducing agent Some prokaryotes use anaerobic respiration, a process that: a. Does not involve an electron transport chain b. Produces ATP solely by substrate-level phosphorylation c. Uses a substance other than oxygen as the final electron acceptor d. Doe ...
Slide 1
... – Energy yield depends on length of carbon chain (ex. 16C palmitic acid results in 129 ATPs, ~3.5x more than glucose) – Ketoacidosis: results if oxaloacetate in short supply; acetyl-CoA converted into ketones, which are weak acids; can occur due to starvation, low-carbohydrate diet, or by uncontroll ...
... – Energy yield depends on length of carbon chain (ex. 16C palmitic acid results in 129 ATPs, ~3.5x more than glucose) – Ketoacidosis: results if oxaloacetate in short supply; acetyl-CoA converted into ketones, which are weak acids; can occur due to starvation, low-carbohydrate diet, or by uncontroll ...
General Biology I (BIOLS 102)
... Occurs universally in organisms (i.e. most likely evolved before the citric acid cycle and the electron transport system) ...
... Occurs universally in organisms (i.e. most likely evolved before the citric acid cycle and the electron transport system) ...
key
... 5. competitive inhibitors – a molecule that is very close to the shape of the true substrate may bind with the enzyme and inhibit the reaction because only binding between the true substrate and enzyme makes the appropriate products a. example – carbon monoxide and carbon dioxide. Carbon monoxide is ...
... 5. competitive inhibitors – a molecule that is very close to the shape of the true substrate may bind with the enzyme and inhibit the reaction because only binding between the true substrate and enzyme makes the appropriate products a. example – carbon monoxide and carbon dioxide. Carbon monoxide is ...
Introduction to Metabolism
... Enzymes are the basic units of metabolism. The substrates of these enzymes are called metabolites. A metabolic pathway is a series of connected enzymatic reactions that produce a specific product. ...
... Enzymes are the basic units of metabolism. The substrates of these enzymes are called metabolites. A metabolic pathway is a series of connected enzymatic reactions that produce a specific product. ...
Lactic acid fermentation
... then be used to drive processes requiring energy, including biosynthesis, locomotion or transportation of molecules across cell membranes… etc. ...
... then be used to drive processes requiring energy, including biosynthesis, locomotion or transportation of molecules across cell membranes… etc. ...
in the presence of oxygen
... • ATP synthase rotates and adds a phosphate group to ADP to make ATP. ...
... • ATP synthase rotates and adds a phosphate group to ADP to make ATP. ...
IB BIOLOGY: Respiration Notes - NatronaBiology-IB2
... sites that create ATP by binding ADP with inorganic phosphate molecules. The result is 36 ATP produced by oxidative phosphorylation. Explain aerobic respiration including the link reaction, the Krebs cycle, the role of NADH +H+, the electron transport chain and the role of oxygen. In aerobic respira ...
... sites that create ATP by binding ADP with inorganic phosphate molecules. The result is 36 ATP produced by oxidative phosphorylation. Explain aerobic respiration including the link reaction, the Krebs cycle, the role of NADH +H+, the electron transport chain and the role of oxygen. In aerobic respira ...
Pentose Phosphate Pathway (aka Hexose monophosphate shunt)
... A second transketolase catalyzes the transfer of C2 from Xu-5-P to E-4-P forming a second F-6-P and GAP. Requires TPP as cofactor Goes through a TPP-Xu5-P adduct as intermediate ...
... A second transketolase catalyzes the transfer of C2 from Xu-5-P to E-4-P forming a second F-6-P and GAP. Requires TPP as cofactor Goes through a TPP-Xu5-P adduct as intermediate ...
Enzymology - Angelfire
... 1959 by Daniel Koshland. It suggested that the active site may not necessarily be exactly of the same shape as the substrate. But the enzyme can have an active site that attracts the substrate. When a substrate combines with an enzyme, it induces a change in the enzyme structure. The amino acids whi ...
... 1959 by Daniel Koshland. It suggested that the active site may not necessarily be exactly of the same shape as the substrate. But the enzyme can have an active site that attracts the substrate. When a substrate combines with an enzyme, it induces a change in the enzyme structure. The amino acids whi ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.