K m - kois.sk
... - chemicals that reduce the rate of enzymic reactions - usually specific, they work at low concentrations - block the enzyme but they do not usually destroy it Irreversible inhibitors: Combine irreversibly with the functional groups of the amino acids in the active site, e.g. nerve gases and pestici ...
... - chemicals that reduce the rate of enzymic reactions - usually specific, they work at low concentrations - block the enzyme but they do not usually destroy it Irreversible inhibitors: Combine irreversibly with the functional groups of the amino acids in the active site, e.g. nerve gases and pestici ...
Lab 6 Enzymes
... interactions. Side chains of amino acids in the active site catalyze the conversion from a substrate to a product. Once the product is formed, it is released from the active site of the enzyme. The catalytic cycle of an enzyme is illustrated below, using sucrase as an ...
... interactions. Side chains of amino acids in the active site catalyze the conversion from a substrate to a product. Once the product is formed, it is released from the active site of the enzyme. The catalytic cycle of an enzyme is illustrated below, using sucrase as an ...
Exercise 7. Enzyme Kinetics
... mechanisms may also yield a rate expression in the Michaelis-Menten form; kcat may then be a ...
... mechanisms may also yield a rate expression in the Michaelis-Menten form; kcat may then be a ...
Enzymes
... Active Site • It is a pocket or cleft in an enzyme molecule. It contains amino acid that create a three dimentional surface. • The amino acids present in the active site (catalytic amino acids) may be far away from each other in the primary structure of the enzyme. • Active site contains a machnery ...
... Active Site • It is a pocket or cleft in an enzyme molecule. It contains amino acid that create a three dimentional surface. • The amino acids present in the active site (catalytic amino acids) may be far away from each other in the primary structure of the enzyme. • Active site contains a machnery ...
enzyme kinetics
... Looking at this plot, we see that V varies linearly with [S] for small [S]. As [S] increases, V “plateaus” indicating that V becomes independent of [S] at large values of [S]. The simplest model which accounts for this behavior is: ...
... Looking at this plot, we see that V varies linearly with [S] for small [S]. As [S] increases, V “plateaus” indicating that V becomes independent of [S] at large values of [S]. The simplest model which accounts for this behavior is: ...
Enzymology Part 2
... 2. Kinetic pattern looks like non-competitive inhibition (net effect is a loss of active enzyme): Vmax decreases 3. Reaction is time dependent decrease in enzymatic activity ie not instantaneous as seen in non competitive inhibition 4. Penicillin is an irreversible inhibitor: binds to serine residue ...
... 2. Kinetic pattern looks like non-competitive inhibition (net effect is a loss of active enzyme): Vmax decreases 3. Reaction is time dependent decrease in enzymatic activity ie not instantaneous as seen in non competitive inhibition 4. Penicillin is an irreversible inhibitor: binds to serine residue ...
Enzymes: Biological Catalysts
... 1. Polypeptide substrate binding. 2. Proton transfer from Ser to His. The substrate forms a tetrahedral transition state with the enzyme. 3. Proton transfer to the C-terminal fragment, which is released by cleavage of the C-N bond. The N-terminal peptide is bound through acyl linkage to serine. 4. A ...
... 1. Polypeptide substrate binding. 2. Proton transfer from Ser to His. The substrate forms a tetrahedral transition state with the enzyme. 3. Proton transfer to the C-terminal fragment, which is released by cleavage of the C-N bond. The N-terminal peptide is bound through acyl linkage to serine. 4. A ...
Course Notes
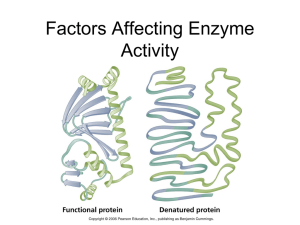
... Denaturation of an enzyme = alters active site so that substrate can no longer fit. Therefore, less ES complexes can be formed and fewer products produced Enzyme Denaturation Factors a. Heavy Metals - e.g. Lead and Mercury- These bond with the protein in enzymes and thereby inactivate them. ...
... Denaturation of an enzyme = alters active site so that substrate can no longer fit. Therefore, less ES complexes can be formed and fewer products produced Enzyme Denaturation Factors a. Heavy Metals - e.g. Lead and Mercury- These bond with the protein in enzymes and thereby inactivate them. ...
Enzyme structure and function
... 1. A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. 2. An enzyme speeds up the rate of a specific reaction, without being used up. 3. What does each enzyme do? Complete the sentences about specific enzymes. a. Lipase br ...
... 1. A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. 2. An enzyme speeds up the rate of a specific reaction, without being used up. 3. What does each enzyme do? Complete the sentences about specific enzymes. a. Lipase br ...
Enzyme Quiz - BiologySemester57
... 14. Alcohols compose a very large family of organic molecules. The alcohol found in beer, wine and distilled spirits is ethanol. The breakdown of ethanol in humans begins with an enzyme called alcohol dehydrogenase, which is found in the liver. Accidental ingestion of methanol, an alcohol that is po ...
... 14. Alcohols compose a very large family of organic molecules. The alcohol found in beer, wine and distilled spirits is ethanol. The breakdown of ethanol in humans begins with an enzyme called alcohol dehydrogenase, which is found in the liver. Accidental ingestion of methanol, an alcohol that is po ...
How Enzymes Work - Manhasset Public Schools
... Gastric juice is active at a pH extending from 0 to 12. Acids have a pH greater than 7. ...
... Gastric juice is active at a pH extending from 0 to 12. Acids have a pH greater than 7. ...
Both PS 7 and PS 8 are due next Thursday
... Which of the following graphs shows the results of reaction rate vs substrate concentration for an non-allosteric enzyme in the absence and presence of a noncompetitive inhibitor (non-competitive inhibitors bind to an enzyme at a site different than the active site)? ...
... Which of the following graphs shows the results of reaction rate vs substrate concentration for an non-allosteric enzyme in the absence and presence of a noncompetitive inhibitor (non-competitive inhibitors bind to an enzyme at a site different than the active site)? ...
How Enzymes Work - Manhasset Public Schools
... Gastric juice is active at a pH extending from 0 to 12. Acids have a pH greater than 7. ...
... Gastric juice is active at a pH extending from 0 to 12. Acids have a pH greater than 7. ...
File
... activity of all the active sites on the enzyme Animation: “ An example of allosteric activation of an ...
... activity of all the active sites on the enzyme Animation: “ An example of allosteric activation of an ...
enzymes - SD57 Mail
... • What would you expect to be the optimal temperature of enzymes that function in the human body? ...
... • What would you expect to be the optimal temperature of enzymes that function in the human body? ...
SG 4,5,6,11
... energy. What is the equation that relates these 3 functions? What are Standard Sate Conditions for non-biological reactions? What are they for biological reactions? Define spontaneous reaction, non-spontaneous reaction, exergonic reaction, endergonic reaction. What is the equation that relates chang ...
... energy. What is the equation that relates these 3 functions? What are Standard Sate Conditions for non-biological reactions? What are they for biological reactions? Define spontaneous reaction, non-spontaneous reaction, exergonic reaction, endergonic reaction. What is the equation that relates chang ...
Chemical Reactions
... chemical reaction, atoms are not created or destroyed – just rearranged. Therefore, chemical equations must be balanced so there is the same number of atoms on both sides of the equation. ...
... chemical reaction, atoms are not created or destroyed – just rearranged. Therefore, chemical equations must be balanced so there is the same number of atoms on both sides of the equation. ...
UB Chapter 3: Enzymes (Exercises 3.6) p.44
... ribonuclease. Since the active site of ribonuclease has been damaged, the enzyme is inactivated. c. i. Competitive inhibition is the result of adding a molecule that has similar structure to the substrate to slow down an enzyme-catalyzed reaction. The inhibitor reduces the chance of the substrate fi ...
... ribonuclease. Since the active site of ribonuclease has been damaged, the enzyme is inactivated. c. i. Competitive inhibition is the result of adding a molecule that has similar structure to the substrate to slow down an enzyme-catalyzed reaction. The inhibitor reduces the chance of the substrate fi ...
General Biology I (BIOLS 102)
... It provides a common energy currency used in many types of reactions When ATP becomes ADP + ℗ the amount of energy released is sufficient for biological function with little waste of energy (~ 7.3 kcal per molecule) ATP breakdown can be coupled to endergonic reactions ...
... It provides a common energy currency used in many types of reactions When ATP becomes ADP + ℗ the amount of energy released is sufficient for biological function with little waste of energy (~ 7.3 kcal per molecule) ATP breakdown can be coupled to endergonic reactions ...
Metabolism of disaccharides: Fructose and Galactose
... • Cause: due to deficiency of fructokinase enzyme • Effect: not serious condition. The excess accumulated fructose is lost in urine 2. Fructose 1,6 biphosphatase deficiency It leads to accumulation of fructose 1,6 biphosphate which inhibits phosphorylase enzyme (glycogenolysis) and fasting ...
... • Cause: due to deficiency of fructokinase enzyme • Effect: not serious condition. The excess accumulated fructose is lost in urine 2. Fructose 1,6 biphosphatase deficiency It leads to accumulation of fructose 1,6 biphosphate which inhibits phosphorylase enzyme (glycogenolysis) and fasting ...
Enzymes
... Feed back inhibition: End-product of pathway inhibits activity of first enzyme (pacemaker enzyme) in pathway: Enzyme's synthesis not affected: Activity of enzyme controlled: ...
... Feed back inhibition: End-product of pathway inhibits activity of first enzyme (pacemaker enzyme) in pathway: Enzyme's synthesis not affected: Activity of enzyme controlled: ...
What are enzymes and how do they work
... 12. How does reaction speed change when the active site is changed? It slows down ...
... 12. How does reaction speed change when the active site is changed? It slows down ...
AP Lab 13: Enzyme Activity
... Background: Enzymes are the catalysts of biological systems. They speed up chemical reactions in biological systems by lowering the activation energy, the energy needed for molecules to begin reacting with each other. Enzymes do this by forming an enzyme-substrate complex that reduces energy require ...
... Background: Enzymes are the catalysts of biological systems. They speed up chemical reactions in biological systems by lowering the activation energy, the energy needed for molecules to begin reacting with each other. Enzymes do this by forming an enzyme-substrate complex that reduces energy require ...
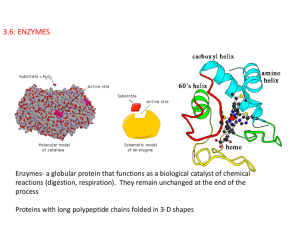
3.6: ENZYMES
... changes in pH (from optimum pH) decrease activity/effectiveness; pH slightly changes the shape of the active site / tertiary structure of protein changed / enzyme denatured; more difficult to form enzyme-substrate complex; [2 max] ...
... changes in pH (from optimum pH) decrease activity/effectiveness; pH slightly changes the shape of the active site / tertiary structure of protein changed / enzyme denatured; more difficult to form enzyme-substrate complex; [2 max] ...
Project: Create Your Own Enzyme!
... lipase breaks down lipids, sucrose breaks down sucrose, and lactase breaks down lactose. But enzymes do more than just breaking down molecules. Some enzymes are also required to build molecules. There are many enzymes in ribosomes (such as peptydil synthetase) that are responsible for building new p ...
... lipase breaks down lipids, sucrose breaks down sucrose, and lactase breaks down lactose. But enzymes do more than just breaking down molecules. Some enzymes are also required to build molecules. There are many enzymes in ribosomes (such as peptydil synthetase) that are responsible for building new p ...
Isomerase

Isomerases are a general class of enzymes which convert a molecule from one isomer to another. Isomerases can either facilitate intramolecular rearrangements in which bonds are broken and formed or they can catalyze conformational changes. The general form of such a reaction is as follows:A–B → B–AThere is only one substrate yielding one product. This product has the same molecular formula as the substrate but differs in bond connectivity or spatial arrangements. Isomerases catalyze reactions across many biological processes, such as in glycolysis and carbohydrate metabolism.