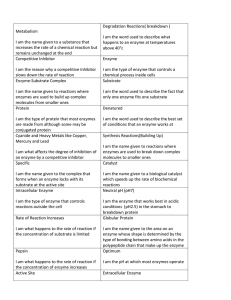
Factors affecting Enzyme Activity
... The activity of an Enzyme is affected by its environmental conditions. Changing these alter the rate of reaction caused by the enzyme. In nature, organisms adjust the conditions of their enzymes to produce anOptimum rate of reaction, where necessary, or they may have enzymes which are adapted to fun ...
... The activity of an Enzyme is affected by its environmental conditions. Changing these alter the rate of reaction caused by the enzyme. In nature, organisms adjust the conditions of their enzymes to produce anOptimum rate of reaction, where necessary, or they may have enzymes which are adapted to fun ...
Chapter 2 I am - Mrs Smith`s Biology
... I am what happens to the rate of reaction if the concentration of enzyme increases Active Site ...
... I am what happens to the rate of reaction if the concentration of enzyme increases Active Site ...
Enzymes are NOT reactants!
... b) [Non-competitive inhibitors]: End product binds at a allosteric (different site), changes shape of active site. Substrate can't bind. ...
... b) [Non-competitive inhibitors]: End product binds at a allosteric (different site), changes shape of active site. Substrate can't bind. ...
Enzyme Introductory Lecture
... The activation energy for these substrates to bind together has been lowered by the enzyme. ...
... The activation energy for these substrates to bind together has been lowered by the enzyme. ...
Enzymes! - Mrs. Ahrens` Science Site
... • The(se) substrate(s) are the reactants that are catalyzed by the enzyme. – Example: Typically something we ingest that needs to be broken down further for use by the body like Lactose from Cow’s milk. (Done by Lactase) ...
... • The(se) substrate(s) are the reactants that are catalyzed by the enzyme. – Example: Typically something we ingest that needs to be broken down further for use by the body like Lactose from Cow’s milk. (Done by Lactase) ...
Enzyme Wi(Re) - Honors Bio
... 15. Which statement is NOT true about the effects of various conditions on the activity of an enzyme? a. Higher temperatures generally increase the activity of an enzyme up to a point b. Above a certain range of temperatures, the protein of an enzyme is denatured c. A change in pH can cause an enzym ...
... 15. Which statement is NOT true about the effects of various conditions on the activity of an enzyme? a. Higher temperatures generally increase the activity of an enzyme up to a point b. Above a certain range of temperatures, the protein of an enzyme is denatured c. A change in pH can cause an enzym ...
Enzymes A simulation of Invertase Activity
... that releases more energy than is used by that building reaction in order for it to take place. However, even when reactions are spontaneous, they often do not occur at a rate sufficient to maintain life. Thus some sort of catalyst, a rate enhancing molecule is needed to promote life functions. This ...
... that releases more energy than is used by that building reaction in order for it to take place. However, even when reactions are spontaneous, they often do not occur at a rate sufficient to maintain life. Thus some sort of catalyst, a rate enhancing molecule is needed to promote life functions. This ...
Enzymes and Protein Structure
... - Enzyme types by reaction catalyzed: - Oxidoreductase (oxidation/reduction, e.g. HRP) - Transferase (transfer of a reactive group, e.g. DNA methyltransferase) - Hydrolase (hydrolysis of a bond, e.g. proteases) - Lyase (non-oxidative, non-hydrolytic bond making/breaking, e.g. adenylyl cyclase (cAMP) ...
... - Enzyme types by reaction catalyzed: - Oxidoreductase (oxidation/reduction, e.g. HRP) - Transferase (transfer of a reactive group, e.g. DNA methyltransferase) - Hydrolase (hydrolysis of a bond, e.g. proteases) - Lyase (non-oxidative, non-hydrolytic bond making/breaking, e.g. adenylyl cyclase (cAMP) ...
Active yet responsive approximately equal to the substrate con- centration normally K
... and the horizontal intercept in a plot of V0 versus V0/[S]? 4. Competing substrates. Suppose that two substrates, A and B, compete for an enzyme. Derive an expression relat- ing the ratio of the rates of utilization of A and B, VA/VB, to the concentrations of these substrates and their values of kc ...
... and the horizontal intercept in a plot of V0 versus V0/[S]? 4. Competing substrates. Suppose that two substrates, A and B, compete for an enzyme. Derive an expression relat- ing the ratio of the rates of utilization of A and B, VA/VB, to the concentrations of these substrates and their values of kc ...
Biochem Flip flop
... A carbohydrate that is made up of long chains of monosaccharides (or simple sugars) is called a polysaccharide. Polysaccharides can be broken down by living organisms into glucose molecules. Remember that glucose is the main energy source for most living things. They must get glucose one way or anot ...
... A carbohydrate that is made up of long chains of monosaccharides (or simple sugars) is called a polysaccharide. Polysaccharides can be broken down by living organisms into glucose molecules. Remember that glucose is the main energy source for most living things. They must get glucose one way or anot ...
ENZYMES A CATALYST is a substance that speeds up a chemical
... chemical reactions that they expedite – so they can be reused over and over again. “LOCK AND KEY” MODEL OF ENZYME ACTION When you go home at night and the door is locked, can it open itself? Nope. You need a key that is just the right shape to fit in that lock. Otherwise you're stuck in the cold. En ...
... chemical reactions that they expedite – so they can be reused over and over again. “LOCK AND KEY” MODEL OF ENZYME ACTION When you go home at night and the door is locked, can it open itself? Nope. You need a key that is just the right shape to fit in that lock. Otherwise you're stuck in the cold. En ...
Topic 15 Carbohydrates
... bloodstream the food molecules must be broken up into small enough units. For example Starch must be broken into Glucose molecules. ...
... bloodstream the food molecules must be broken up into small enough units. For example Starch must be broken into Glucose molecules. ...
j-catalysts-2
... Many digestive enzymes (biological catalysts) which catalyze the hydrolysis of proteins, fats, and carbohydrates in foods also use acid/base catalysis where the acids and bases come from specific _______________ _____________ side chains. 2) NO catalyzing the production of _______________ in the lo ...
... Many digestive enzymes (biological catalysts) which catalyze the hydrolysis of proteins, fats, and carbohydrates in foods also use acid/base catalysis where the acids and bases come from specific _______________ _____________ side chains. 2) NO catalyzing the production of _______________ in the lo ...
1. Vmax, the maximum velocity, of an enzyme-catalyzed
... d. irreversible inhibitor. 4. Diisopropyl fluorophosphate (DIFP) inactivates chymotrypsin by covalently modifying serine-195. This occurs because a. ...
... d. irreversible inhibitor. 4. Diisopropyl fluorophosphate (DIFP) inactivates chymotrypsin by covalently modifying serine-195. This occurs because a. ...
Lesson 5: Enzymes
... 1. Extreme Temperature are the most dangerous - high temps may denature (unfold) the enzyme. 2. pH (most like 6 - 8 pH near neutral) 3. Ionic concentration (salt ions) ...
... 1. Extreme Temperature are the most dangerous - high temps may denature (unfold) the enzyme. 2. pH (most like 6 - 8 pH near neutral) 3. Ionic concentration (salt ions) ...
Bchm2000_P3 v1 - U of L Class Index
... (a) What is the most likely inside diameter of 'U' shaped DNA binding proteins? (b) Would you expect 'U' shaped DNA binding proteins to bind to dsRNA with an equivalent sequence? Explain. ...
... (a) What is the most likely inside diameter of 'U' shaped DNA binding proteins? (b) Would you expect 'U' shaped DNA binding proteins to bind to dsRNA with an equivalent sequence? Explain. ...
ARABINANASE ACTIVITY IN PECT
... arabinofuranosyl residues, leaving linear 1,5- α -arabinan. This ...
... arabinofuranosyl residues, leaving linear 1,5- α -arabinan. This ...
File
... model, however, failed to explain many other behavioural features of enzymes such as, high enzyme specificity. ...
... model, however, failed to explain many other behavioural features of enzymes such as, high enzyme specificity. ...
Enzymes
... • Enzymes LOWER the ACTIVATION ENERGY IN A CHEMICAL REACTION that’s why the reaction goes faster. ...
... • Enzymes LOWER the ACTIVATION ENERGY IN A CHEMICAL REACTION that’s why the reaction goes faster. ...
BIOCHEMISTRY (CHEM 360)
... It is popularly accepted that high doses of vitamin C prevent the common cold (although not clinically/biochemically proven); whereas high doses of Vitamin A could be quite hazardous. Explain why high doses of Vitamin C are tolerable, even if there are no proven benefits; on the other hand excessive ...
... It is popularly accepted that high doses of vitamin C prevent the common cold (although not clinically/biochemically proven); whereas high doses of Vitamin A could be quite hazardous. Explain why high doses of Vitamin C are tolerable, even if there are no proven benefits; on the other hand excessive ...
Chymosin Lab
... The stages of enzyme catalysis • Substrate(s) binding • Reaction of substrate to form product(s) • Release of products • The enzyme is ready to bind the next substrate • Enzymes are unchanged by the reactions they catalyze ...
... The stages of enzyme catalysis • Substrate(s) binding • Reaction of substrate to form product(s) • Release of products • The enzyme is ready to bind the next substrate • Enzymes are unchanged by the reactions they catalyze ...
Enzyme - fiveless|notes
... products no longer fit into active site, escapes into surrounding medium active site free to receive further substrate molecules ...
... products no longer fit into active site, escapes into surrounding medium active site free to receive further substrate molecules ...
Isomerase

Isomerases are a general class of enzymes which convert a molecule from one isomer to another. Isomerases can either facilitate intramolecular rearrangements in which bonds are broken and formed or they can catalyze conformational changes. The general form of such a reaction is as follows:A–B → B–AThere is only one substrate yielding one product. This product has the same molecular formula as the substrate but differs in bond connectivity or spatial arrangements. Isomerases catalyze reactions across many biological processes, such as in glycolysis and carbohydrate metabolism.