Word document
... 39. In humans what is the path of an oxygen molecule from the mouth to the alveoli 40. Explain what happens to an enzyme molecule when it is denatured by high temperatures. 41. Why does an enzyme only speed up one (or at the most a few) different chemical reaction(s)? 42. How do enzymes speed up the ...
... 39. In humans what is the path of an oxygen molecule from the mouth to the alveoli 40. Explain what happens to an enzyme molecule when it is denatured by high temperatures. 41. Why does an enzyme only speed up one (or at the most a few) different chemical reaction(s)? 42. How do enzymes speed up the ...
CHapter 2b Practice problems answers
... 3. The ability to move or change matter is _____ energy _______________. 4. All living things require a source of __ energy __________________ to carry out their life activities. 5. The starting materials for chemical reactions are called _____reactants_______________, while the new substances that ...
... 3. The ability to move or change matter is _____ energy _______________. 4. All living things require a source of __ energy __________________ to carry out their life activities. 5. The starting materials for chemical reactions are called _____reactants_______________, while the new substances that ...
DO NOW Monday 2/12
... Large molecules in food are broken down into smaller molecules to: – Provide energy to the body – Provide building blocks to make new molecules ...
... Large molecules in food are broken down into smaller molecules to: – Provide energy to the body – Provide building blocks to make new molecules ...
UNIT 1 PART 1: THE SCIENTIFIC METHOD
... Mammals, insects, birds, sponges Can move on their own Multicellular, cells do not have cell walls Eukaryotic Must get food from the environment ...
... Mammals, insects, birds, sponges Can move on their own Multicellular, cells do not have cell walls Eukaryotic Must get food from the environment ...
this lecture as PDF here
... Enzymes undergo physical changes during the reaction but revert to their original form at the end of the reaction. ...
... Enzymes undergo physical changes during the reaction but revert to their original form at the end of the reaction. ...
Enzymes
... 2. Enzymes are catalysts that speed up reactions (they turn substrates into products) 3. Enzymes are specific (only 1 enzyme works for 1 substrate) 4. Enzymes do not get used up in the reaction 5. Enzymes only work at a specific temperature and pH. 6. Enzymes end in -ase ...
... 2. Enzymes are catalysts that speed up reactions (they turn substrates into products) 3. Enzymes are specific (only 1 enzyme works for 1 substrate) 4. Enzymes do not get used up in the reaction 5. Enzymes only work at a specific temperature and pH. 6. Enzymes end in -ase ...
SOL Review * Chemistry/Macromolecules
... Name the three particles that make up and atom: _______________________________________________ Define and give an example for: Physical change: _________________________________________________________________________ Chemical change: ________________________________________________________________ ...
... Name the three particles that make up and atom: _______________________________________________ Define and give an example for: Physical change: _________________________________________________________________________ Chemical change: ________________________________________________________________ ...
Energy/Chemical Energy in the Cell Chapter 5
... pathway of lower activation energy – neither changes the position of equilibrium or yield of the reaction • enzymes are highly specific for their substrate, whereas inorganic catalysts are often non-specific and can catalyze several reactions • enzymes have an optimum temperature and are denatured a ...
... pathway of lower activation energy – neither changes the position of equilibrium or yield of the reaction • enzymes are highly specific for their substrate, whereas inorganic catalysts are often non-specific and can catalyze several reactions • enzymes have an optimum temperature and are denatured a ...
Ecotek Students Improve Protocol for the Enzyme Hydrolysis of Starch
... alternative methods. It can happen when you least expect it. Student scientists, Amber Young, 9th grader and Alisha Boyd, 10th grader, can attest to this fact. While investigating how enzymes affect biochemical processes, the student scientists were able to develop a simple one step process to analy ...
... alternative methods. It can happen when you least expect it. Student scientists, Amber Young, 9th grader and Alisha Boyd, 10th grader, can attest to this fact. While investigating how enzymes affect biochemical processes, the student scientists were able to develop a simple one step process to analy ...
HANDOUT- Enzymes! (Enzyme Reaction Rates)
... Background information for: Pineapple Enzyme Labs 1-3; Paperase Lab ...
... Background information for: Pineapple Enzyme Labs 1-3; Paperase Lab ...
Enzyme Kinetics II
... d. Two substrates will have to become P and Q – the products i. Two substrates in, two products out e. You can see here that it becomes a little more complicated f. In a single displacement reaction, you can see that this is how the Lineweaver-Burk plot looks g. If you keep increasing concentration ...
... d. Two substrates will have to become P and Q – the products i. Two substrates in, two products out e. You can see here that it becomes a little more complicated f. In a single displacement reaction, you can see that this is how the Lineweaver-Burk plot looks g. If you keep increasing concentration ...
biochemistry 14_15 - Pleasantville High School
... speeds up the rate of a chemical reaction without entering the reaction itself • enzymes: organic catalysts made of protein • most enzyme names end in -ase • enzymes lower the energy needed to start a chemical reaction. (activation energy) • begin to be destroyed above 45øC. (above this temperature ...
... speeds up the rate of a chemical reaction without entering the reaction itself • enzymes: organic catalysts made of protein • most enzyme names end in -ase • enzymes lower the energy needed to start a chemical reaction. (activation energy) • begin to be destroyed above 45øC. (above this temperature ...
Section 2-3
... Some Organic molecules have the same CHEMICAL FORMULA but have different 3-D structure Carbon can covalently bond to other atoms of carbon Sharing Two or even Three Pair of Electrons with another Atom A. SINGLE BOND - A bond formed when two atoms share ONE pair of electrons. B. DOUBLE BOND - Atoms s ...
... Some Organic molecules have the same CHEMICAL FORMULA but have different 3-D structure Carbon can covalently bond to other atoms of carbon Sharing Two or even Three Pair of Electrons with another Atom A. SINGLE BOND - A bond formed when two atoms share ONE pair of electrons. B. DOUBLE BOND - Atoms s ...
A group on the enzyme acts as an acid or base
... for catalyst to be regenerated in original form. Examples of general acid/base catalysts among protein functional groups: His imidazole a-amino group thiol of Cys R group carboxyls of Glu, Asp Sidechain amino group of Lys Aromatic OH of Tyr Guanidino group of Arg ...
... for catalyst to be regenerated in original form. Examples of general acid/base catalysts among protein functional groups: His imidazole a-amino group thiol of Cys R group carboxyls of Glu, Asp Sidechain amino group of Lys Aromatic OH of Tyr Guanidino group of Arg ...
The link between Darwin and antioxidants from olives
... for the biosynthesis of commercially-valuable HTyr, from a cheap and abundant substrate, 2phenylethanol (PEA). This enzymatic hydroxylation is a novel and promising method to synthesize HTyr in a low cost single-step reaction, with high selectivity while utilizing an environmentally friendly process ...
... for the biosynthesis of commercially-valuable HTyr, from a cheap and abundant substrate, 2phenylethanol (PEA). This enzymatic hydroxylation is a novel and promising method to synthesize HTyr in a low cost single-step reaction, with high selectivity while utilizing an environmentally friendly process ...
Chapter 6
... – 200 molecules of carbonic acid per hour made without enzyme – 600,000 molecules formed per second with enzyme ...
... – 200 molecules of carbonic acid per hour made without enzyme – 600,000 molecules formed per second with enzyme ...
B. Enzymes have four features
... groups into alignment so that chemical bonds can be broken, created, and rearranged. 2. The substrate is brought to its transition state, the point when a reaction can occur. 3. enzyme + substrate enzyme-substrate complex (intermediate) enzyme + product ...
... groups into alignment so that chemical bonds can be broken, created, and rearranged. 2. The substrate is brought to its transition state, the point when a reaction can occur. 3. enzyme + substrate enzyme-substrate complex (intermediate) enzyme + product ...
Enzymes_loop_game
... What is the name of the area on What are enzymes made up of? the enzyme to which the substrate attaches? ...
... What is the name of the area on What are enzymes made up of? the enzyme to which the substrate attaches? ...
BIOCHEMISTRY
... speeds up the rate of a chemical reaction without entering the reaction itself • enzymes: organic catalysts made of protein • most enzyme names end in -ase • enzymes lower the energy needed to start a chemical reaction. (activation energy) • begin to be destroyed above 45øC. (above this temperature ...
... speeds up the rate of a chemical reaction without entering the reaction itself • enzymes: organic catalysts made of protein • most enzyme names end in -ase • enzymes lower the energy needed to start a chemical reaction. (activation energy) • begin to be destroyed above 45øC. (above this temperature ...
Lecture 1 (BC) - WordPress.com
... One way in which a glucose carrier can be driven by a Na+ gradient. The carrier oscillates between two alternate states, A and B. In the A state, the protein is open to the aextracellular space; in the B state, it is open to the cytosol. Binding of Na+ and glucose is cooperative that is, the bindin ...
... One way in which a glucose carrier can be driven by a Na+ gradient. The carrier oscillates between two alternate states, A and B. In the A state, the protein is open to the aextracellular space; in the B state, it is open to the cytosol. Binding of Na+ and glucose is cooperative that is, the bindin ...
Biochemistry Review
... (water)from the individual molecules so that a bond may form between them and result in a more complex molecule is called ___________ ...
... (water)from the individual molecules so that a bond may form between them and result in a more complex molecule is called ___________ ...
enzymes - onlinebiosurgery
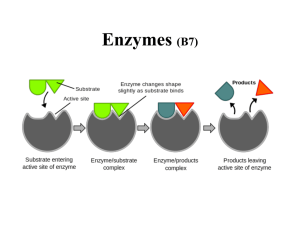
... • This is similar to the ‘lock and key’ but it is thought that the enzyme changes shape slightly as substrate binds i.e. hand fitting into a glove ...
... • This is similar to the ‘lock and key’ but it is thought that the enzyme changes shape slightly as substrate binds i.e. hand fitting into a glove ...
Enzymes - Madison County Schools
... Equilibrium eventually reached b/c all the substrate is being broken down and adding more enzymes will not affect the reaction rate (Because those enzymes will have no substrate to break down). ...
... Equilibrium eventually reached b/c all the substrate is being broken down and adding more enzymes will not affect the reaction rate (Because those enzymes will have no substrate to break down). ...
can
... unsuccessful. • Once the crystal structure was available, it was decided to search for transition state analogs. • Recall that the active site of the enzyme will bind and stabilize the transition state more effectively than it will stabilize the substrate itself, thus resulting in an overall decreas ...
... unsuccessful. • Once the crystal structure was available, it was decided to search for transition state analogs. • Recall that the active site of the enzyme will bind and stabilize the transition state more effectively than it will stabilize the substrate itself, thus resulting in an overall decreas ...
Isomerase

Isomerases are a general class of enzymes which convert a molecule from one isomer to another. Isomerases can either facilitate intramolecular rearrangements in which bonds are broken and formed or they can catalyze conformational changes. The general form of such a reaction is as follows:A–B → B–AThere is only one substrate yielding one product. This product has the same molecular formula as the substrate but differs in bond connectivity or spatial arrangements. Isomerases catalyze reactions across many biological processes, such as in glycolysis and carbohydrate metabolism.