enzyme - iGEM 2014
... • An increased interaction of the enzyme and substrate occurs in the transition-state (ES‡) • The enzyme distorts the substrate, forcing it toward the transition state • An enzyme must be complementary to the transition-state in shape and chemical character • Enzymes may bind their transition states ...
... • An increased interaction of the enzyme and substrate occurs in the transition-state (ES‡) • The enzyme distorts the substrate, forcing it toward the transition state • An enzyme must be complementary to the transition-state in shape and chemical character • Enzymes may bind their transition states ...
Enzymes - Fairfield Public Schools
... diagram is the substrate? Explain. 4. At which step does the chemical reaction actually take place? 5. What chemical reaction is catalyzed by the enzyme? 6. How can you tell from the diagram that sucrase is not used up in the reaction? ...
... diagram is the substrate? Explain. 4. At which step does the chemical reaction actually take place? 5. What chemical reaction is catalyzed by the enzyme? 6. How can you tell from the diagram that sucrase is not used up in the reaction? ...
Clinical biochemistry (9) Enzymes and isoenzymes
... In most enzymatic reactions ,however, two (and some times more) different substrate molecules bind to the enzyme and participate in reaction. (a) 2 products formed together. (b) Products formed one after another. ...
... In most enzymatic reactions ,however, two (and some times more) different substrate molecules bind to the enzyme and participate in reaction. (a) 2 products formed together. (b) Products formed one after another. ...
Chapter 16.6 & 16.7 Enzymes & Enzyme Actions
... Speed up chemical reactions Small amount needed to catalyse a reaction because enzymes can be used again and again The shapes of the active sites make enzymes highly specific, meaning they can only interact with 1 type of substrate to form an enzyme-substrate complex ...
... Speed up chemical reactions Small amount needed to catalyse a reaction because enzymes can be used again and again The shapes of the active sites make enzymes highly specific, meaning they can only interact with 1 type of substrate to form an enzyme-substrate complex ...
2. Enzyme activity - Lectures For UG-5
... • Enzymes concentrations are always performed in zeroorder kinetics with substrate in sufficient excess to ensure that not more than 20% of the available substrate is converted to product. • Any coenzymes also must be in excess. • NAD or NADH is often convenient as a reagent for a coupled- enzyme as ...
... • Enzymes concentrations are always performed in zeroorder kinetics with substrate in sufficient excess to ensure that not more than 20% of the available substrate is converted to product. • Any coenzymes also must be in excess. • NAD or NADH is often convenient as a reagent for a coupled- enzyme as ...
Carbon and Molecular Diversity
... • Carbon frequently combines with O, H, and N. • Learn the valence numbers for all 4 of these atoms! ...
... • Carbon frequently combines with O, H, and N. • Learn the valence numbers for all 4 of these atoms! ...
14-7-SA-V1-S1__enzym..
... b. typically yields product more rapidly with an enzyme than the normal substrate. c. is less stable when binding to an enzyme than the normal substrate. d. stabilizes the transition state for the normal enzyme-substrate complex. ...
... b. typically yields product more rapidly with an enzyme than the normal substrate. c. is less stable when binding to an enzyme than the normal substrate. d. stabilizes the transition state for the normal enzyme-substrate complex. ...
Enzyme Substrate Reactions
... 2. At high temperatures, the rate of enzyme action decreases because the increased heat (1.) increases the concentration of the enzyme (3.) alters the active site of the enzyme (2.) changes the pH of the system (4.) neutralizes the acids and bases 3. Enzymes influence chemical reactions in living sy ...
... 2. At high temperatures, the rate of enzyme action decreases because the increased heat (1.) increases the concentration of the enzyme (3.) alters the active site of the enzyme (2.) changes the pH of the system (4.) neutralizes the acids and bases 3. Enzymes influence chemical reactions in living sy ...
Enzymes and Activation Energy
... relationships between temperature and enzyme activity for a human and for a thermophilic (heat loving) bacteria. • Notice the optimum (best) activity level for each enzyme matches the environment that the enzyme has to work in. • Human enzymes generally work best at our temperature (37°C) while the ...
... relationships between temperature and enzyme activity for a human and for a thermophilic (heat loving) bacteria. • Notice the optimum (best) activity level for each enzyme matches the environment that the enzyme has to work in. • Human enzymes generally work best at our temperature (37°C) while the ...
Purves, W. (2004) Life, the science of biology. 7th
... small amount of energy along with alcohol or lactic acid (Silberstein, 2011). Even though organisms make much more energy from the reactions processed in oxygen, many higher species have developed a way to live for a short period of time even when oxygen is low. Many plants are even more adept at th ...
... small amount of energy along with alcohol or lactic acid (Silberstein, 2011). Even though organisms make much more energy from the reactions processed in oxygen, many higher species have developed a way to live for a short period of time even when oxygen is low. Many plants are even more adept at th ...
Cell Physiology
... Binding of a molecule to a site other than the active site may result in an enzyme conformational change that either turns the enzyme “on or off” If the modulator is bound by non-covalent forces; it is allosteric modulation (the most common type); if bound covalently, it is covalent modulation (wh ...
... Binding of a molecule to a site other than the active site may result in an enzyme conformational change that either turns the enzyme “on or off” If the modulator is bound by non-covalent forces; it is allosteric modulation (the most common type); if bound covalently, it is covalent modulation (wh ...
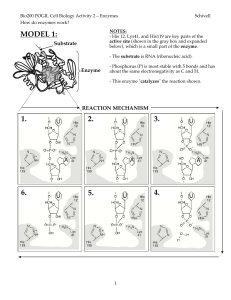
Class3 POGIL Enzyme Mechanics Worksheet
... b. Which mutant helps to answer this question? ________ 14. When the transfer of a proton between the enzyme and substrate is prevented, is the reaction rate changed slightly or dramatically? __________ 15. Even if an R-group (that is part of the active site) does not normally accept or donate proto ...
... b. Which mutant helps to answer this question? ________ 14. When the transfer of a proton between the enzyme and substrate is prevented, is the reaction rate changed slightly or dramatically? __________ 15. Even if an R-group (that is part of the active site) does not normally accept or donate proto ...
Study Guide
... protein structure and how they contribute to the overall 3D structure of the protein molecule. ...
... protein structure and how they contribute to the overall 3D structure of the protein molecule. ...
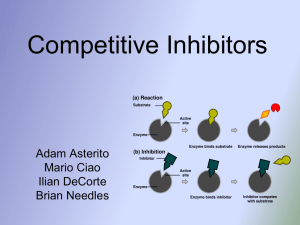
Competitive Inhibitors
... at the active site, where the substrates would normally bind. • This protein has a similar shape of the substrate that binds to the enzyme, it will cause the enzyme to stop working (inhibit). • An inhibitor can be reversible if the concentration of the substrate is increased enough. ...
... at the active site, where the substrates would normally bind. • This protein has a similar shape of the substrate that binds to the enzyme, it will cause the enzyme to stop working (inhibit). • An inhibitor can be reversible if the concentration of the substrate is increased enough. ...
Enzymes - OpenStax CNX
... to occur. Certain chemical reactions might proceed best in a slightly acidic or non-polar environment. The chemical properties that emerge from the particular arrangement of amino acid residues within an active site create the perfect environment for an enzyme's speci c substrates to react. You've l ...
... to occur. Certain chemical reactions might proceed best in a slightly acidic or non-polar environment. The chemical properties that emerge from the particular arrangement of amino acid residues within an active site create the perfect environment for an enzyme's speci c substrates to react. You've l ...
Enzymes - OpenStax CNX
... to occur. Certain chemical reactions might proceed best in a slightly acidic or non-polar environment. The chemical properties that emerge from the particular arrangement of amino acid residues within an active site create the perfect environment for an enzyme's speci c substrates to react. You've l ...
... to occur. Certain chemical reactions might proceed best in a slightly acidic or non-polar environment. The chemical properties that emerge from the particular arrangement of amino acid residues within an active site create the perfect environment for an enzyme's speci c substrates to react. You've l ...
Enzymes
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
Enzymes worksheet
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
Enzymes
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
Organic Chemistry Notes
... Many of the molecules in living cells are very large and known as macromolecules Most macromolecules are formed by a process known as polymerization through which large compounds are built The smaller units or monomers join together to form polymers. Polymers are formed by dehydration synthesis beca ...
... Many of the molecules in living cells are very large and known as macromolecules Most macromolecules are formed by a process known as polymerization through which large compounds are built The smaller units or monomers join together to form polymers. Polymers are formed by dehydration synthesis beca ...
Enzymes
... wililurnto a study of enzyme activity. Take a look at the text below and color the appropriate structures .. Energy must be supplied in order for most cellular chemical reactions to proceed. The initial input of energy required to start a reaction is called the energy of activation. As is shown in t ...
... wililurnto a study of enzyme activity. Take a look at the text below and color the appropriate structures .. Energy must be supplied in order for most cellular chemical reactions to proceed. The initial input of energy required to start a reaction is called the energy of activation. As is shown in t ...
This syllabus was adapted from Introduction to Biochemistry I taught
... In this case, the rate does not increase continuously. Instead, a point is reached after which the rate stays the same even if we increase the substrate concentration further. This happens because, at the saturation point, substrate molecules are bound to all available active sites of the enzymes. ...
... In this case, the rate does not increase continuously. Instead, a point is reached after which the rate stays the same even if we increase the substrate concentration further. This happens because, at the saturation point, substrate molecules are bound to all available active sites of the enzymes. ...
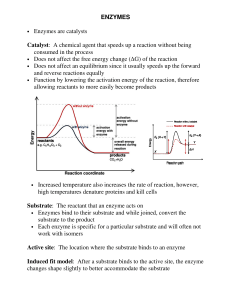
ENZYMES • Enzymes are catalysts Catalyst: A chemical agent that
... • Each enzyme is specific for a particular substrate and will often not work with isomers Active site: The location where the substrate binds to an enzyme Induced fit model: After a substrate binds to the active site, the enzyme changes shape slightly to better accommodate the substrate ...
... • Each enzyme is specific for a particular substrate and will often not work with isomers Active site: The location where the substrate binds to an enzyme Induced fit model: After a substrate binds to the active site, the enzyme changes shape slightly to better accommodate the substrate ...
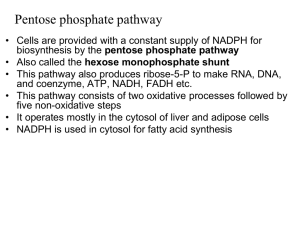
pentose phosphate pathway
... AMP concentration is more sensitive indicator of cell’s energetic state than is [ATP] AMP-activated protein kinase - regulated by [AMP] - A reduced nutrient supply or by increase exercise cause the rise in [AMP] - increase glucose uptake, activates glycolysis and fatty acid oxidation - suppress ener ...
... AMP concentration is more sensitive indicator of cell’s energetic state than is [ATP] AMP-activated protein kinase - regulated by [AMP] - A reduced nutrient supply or by increase exercise cause the rise in [AMP] - increase glucose uptake, activates glycolysis and fatty acid oxidation - suppress ener ...
Isomerase

Isomerases are a general class of enzymes which convert a molecule from one isomer to another. Isomerases can either facilitate intramolecular rearrangements in which bonds are broken and formed or they can catalyze conformational changes. The general form of such a reaction is as follows:A–B → B–AThere is only one substrate yielding one product. This product has the same molecular formula as the substrate but differs in bond connectivity or spatial arrangements. Isomerases catalyze reactions across many biological processes, such as in glycolysis and carbohydrate metabolism.