LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
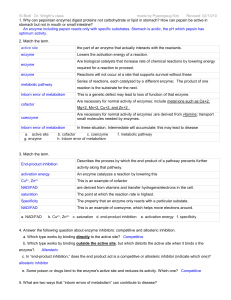
... I. Choose the correct answer: (5 x 1 = 5 marks) (1) The type of inhibition caused by suicide inhibitors is (a) Competitive (b) Non-competitive (c) Uncompetitive (d) Irreversible (2) The number of moles of substrate converted to product per unit time is (a) Enzyme activity (b) Specific activity (c) T ...
... I. Choose the correct answer: (5 x 1 = 5 marks) (1) The type of inhibition caused by suicide inhibitors is (a) Competitive (b) Non-competitive (c) Uncompetitive (d) Irreversible (2) The number of moles of substrate converted to product per unit time is (a) Enzyme activity (b) Specific activity (c) T ...
+ Enzyme Inhibitors
... enzyme out of the thousands of types present in our bodies. E.g., the disease phenylketonuria (PKU) results from a mutation of a single amino acid in the enzyme phenylalanine hydroxylase, which catalyzes the first step in the degradation of phenylalanine The result is a build-up of phenylalanine and ...
... enzyme out of the thousands of types present in our bodies. E.g., the disease phenylketonuria (PKU) results from a mutation of a single amino acid in the enzyme phenylalanine hydroxylase, which catalyzes the first step in the degradation of phenylalanine The result is a build-up of phenylalanine and ...
MECHANISTIC INVESTIGATION OF D-ARGININE DEHYDROGENASE FROM PSEUDOMONAS AERUGINOSA
... the substrates for which the steady state kinetic parameters are available.1,2 Here, the chemical mechanism of leucine oxidation catalyzed by DADH was investigated with pH, substrate, solvent and β-‐secondary ...
... the substrates for which the steady state kinetic parameters are available.1,2 Here, the chemical mechanism of leucine oxidation catalyzed by DADH was investigated with pH, substrate, solvent and β-‐secondary ...
C483 Summer 2015 Exam 2 Name 1. 20 pts Fill in the blanks (2
... essential cofactor for an enzyme that activates thrombin, a protease that induces blood coagulation. (Vitamin K was named after the word “koagulation” by the Danish scientist who discovered it.) In order for vitamin K to function in its catalytic role, it must be oxidized by the enzyme that activate ...
... essential cofactor for an enzyme that activates thrombin, a protease that induces blood coagulation. (Vitamin K was named after the word “koagulation” by the Danish scientist who discovered it.) In order for vitamin K to function in its catalytic role, it must be oxidized by the enzyme that activate ...
Cellular Mechanisms
... product. e.g. • Synthesis of isoleucine from threonine • First step catalysed by threonine ...
... product. e.g. • Synthesis of isoleucine from threonine • First step catalysed by threonine ...
Enzymes
... a. Which type works by binding directly to the active site? Competitive b. Which type works by binding outside the active site, but which distorts the active site when it binds o the enzyme?. ...
... a. Which type works by binding directly to the active site? Competitive b. Which type works by binding outside the active site, but which distorts the active site when it binds o the enzyme?. ...
midterm 2 asnwer scheme
... eg. Ethanol interfere with hydrophobic interaction Detergents – these amphiphatic molecules disrupt hydrophobic interaction causing proteins to unfold into extended polypeptide chains (amphiphatic = contain nonpolar and polar components) Reducing agents – eg. Urea, β-mercaptoethanol, will conver ...
... eg. Ethanol interfere with hydrophobic interaction Detergents – these amphiphatic molecules disrupt hydrophobic interaction causing proteins to unfold into extended polypeptide chains (amphiphatic = contain nonpolar and polar components) Reducing agents – eg. Urea, β-mercaptoethanol, will conver ...
Organic chem and enzyme review game
... and label all parts of the enzyme substrate complex. Include: ...
... and label all parts of the enzyme substrate complex. Include: ...
Enzymes - WordPress.com
... • Temperature – increasing it increases rate of reaction • Temperature coefficient or Q10 is a value for the reaction that shows how much the rate increases when you increase the temperature by 10oC • At temperatures before optimum if the Q10 is 2 then the rate doubles for 10oC increase • A value of ...
... • Temperature – increasing it increases rate of reaction • Temperature coefficient or Q10 is a value for the reaction that shows how much the rate increases when you increase the temperature by 10oC • At temperatures before optimum if the Q10 is 2 then the rate doubles for 10oC increase • A value of ...
Lecture 8: 9/9
... 3. Noncompetitive inhibition: The inhibitor binds either the enzyme or enzyme‐ substrate complex. ...
... 3. Noncompetitive inhibition: The inhibitor binds either the enzyme or enzyme‐ substrate complex. ...
What Are Enzymes?
... How do enzymes work? • Each enzyme has a unique 3-D shape, including a surface groove called an ACTIVE SITE. • One or more molecules called SUBSTRATES chemically bond to the enzyme’s active site. • When joined they are called an ENZYME-SUBSTRATE COMPLEX • Changes in how the atoms are bonded occur r ...
... How do enzymes work? • Each enzyme has a unique 3-D shape, including a surface groove called an ACTIVE SITE. • One or more molecules called SUBSTRATES chemically bond to the enzyme’s active site. • When joined they are called an ENZYME-SUBSTRATE COMPLEX • Changes in how the atoms are bonded occur r ...
Chapter 4
... pH – alters enzyme structure by altering charge Temperature – increases activity by moving molecules closer to the activation energy, and by making ∆G slightly more negative… until the enzyme "denatures" Coenzymes – like biotin in amino group transfer – bind reversibly but participate directly Metal ...
... pH – alters enzyme structure by altering charge Temperature – increases activity by moving molecules closer to the activation energy, and by making ∆G slightly more negative… until the enzyme "denatures" Coenzymes – like biotin in amino group transfer – bind reversibly but participate directly Metal ...
Rate of enzymatic reactions
... reaction increases as the temperature is raised. Higher temperature generally causes more collisions among the molecules and therefore increases the rate of a reaction. More collisions increase the likelihood that substrate will collide with the active site of the enzyme, thus increasing the rate of ...
... reaction increases as the temperature is raised. Higher temperature generally causes more collisions among the molecules and therefore increases the rate of a reaction. More collisions increase the likelihood that substrate will collide with the active site of the enzyme, thus increasing the rate of ...
FACTORS AFFECTING ENZYME ACTION
... EXAM TIP: When answering questions on enzymes, always use the words ‘enzyme-substrate complex’ and ‘active site’, worth 1 mark. ...
... EXAM TIP: When answering questions on enzymes, always use the words ‘enzyme-substrate complex’ and ‘active site’, worth 1 mark. ...
the active site
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
enzyme
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
Enzyme Activity - Model High School
... and enzymes work together like puzzles. A substrate is a chemical that can bond onto a specific enzyme. Only one type of enzyme with lock onto the active site of the substrate chemical (like a puzzle piece). Thus, they are very specific.When the reaction occurs, products are made from the substrate. ...
... and enzymes work together like puzzles. A substrate is a chemical that can bond onto a specific enzyme. Only one type of enzyme with lock onto the active site of the substrate chemical (like a puzzle piece). Thus, they are very specific.When the reaction occurs, products are made from the substrate. ...
Enzymes - WordPress.com
... All enzymes are tertiary globular proteins, where the protein chain is folded back on itself into a spherical or globular shape. Each enzyme has its own sequence of amino acids and is held in its tertiary structure by hydrogen bonds, disulfide bridges and ionic bonds. ...
... All enzymes are tertiary globular proteins, where the protein chain is folded back on itself into a spherical or globular shape. Each enzyme has its own sequence of amino acids and is held in its tertiary structure by hydrogen bonds, disulfide bridges and ionic bonds. ...
ภาพนิ่ง 1
... catalytic step and hence decreases or eliminates enzyme activity (formation of P). Notice in the reciprocal plot, a non-competitive inhibitor does not affect the binding of the substrate (Km), but it does result in a decrease in Vmax. This can be explained by the fact that since inhibitor bound to a ...
... catalytic step and hence decreases or eliminates enzyme activity (formation of P). Notice in the reciprocal plot, a non-competitive inhibitor does not affect the binding of the substrate (Km), but it does result in a decrease in Vmax. This can be explained by the fact that since inhibitor bound to a ...
answers_ch04
... histidine and aspartate. Serine would serve as a nucleophile, histidine as an acid/base catalyst and aspartate as an activating and orientating group. The actual mechanism for the hydrolysis of acetylcholine is described in section 19.16.3.2. ...
... histidine and aspartate. Serine would serve as a nucleophile, histidine as an acid/base catalyst and aspartate as an activating and orientating group. The actual mechanism for the hydrolysis of acetylcholine is described in section 19.16.3.2. ...
Clinical biochemistry (9) Enzymes and isoenzymes
... General properties of the enzymes 1) They are proteinous in nature. 2) They accelerate the rate of the reaction by: (a) not altering the reaction equilibrium (b) being required in minute quantity (c) being not consumed in the overall reaction. 3) They act as catalysts. 4) They are very specific for ...
... General properties of the enzymes 1) They are proteinous in nature. 2) They accelerate the rate of the reaction by: (a) not altering the reaction equilibrium (b) being required in minute quantity (c) being not consumed in the overall reaction. 3) They act as catalysts. 4) They are very specific for ...
Review on Biochemistry: Protein Chemistry
... 10. Reversible inhibitor Inhibitor type Binding site on enzyme Competitive Specifically at the catalytic (active site), inhibitor has a similar structure as the substrate. Inhibition is reversed by substrate Noncompetitive I binds E or ES complex other than the active site. (mixed type) Inhibition ...
... 10. Reversible inhibitor Inhibitor type Binding site on enzyme Competitive Specifically at the catalytic (active site), inhibitor has a similar structure as the substrate. Inhibition is reversed by substrate Noncompetitive I binds E or ES complex other than the active site. (mixed type) Inhibition ...
Enzymes Part 2
... Enzymes fit with their substrate like a lock and key. Forms enzyme substrate complex. ...
... Enzymes fit with their substrate like a lock and key. Forms enzyme substrate complex. ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.