In Anfinsen`s experiment, RNAse was denatured with urea and β
... -Lactamase is an enzyme that conveys resistance to -lactam antibiotics such as penicillins. For penicillin G, the KM for -lactamase is 13 μM. Which of the following plots represents inhibition of the reaction of -lactamase with penicillin G by clavulcanic acid, a competitive inhibitor? (Concent ...
... -Lactamase is an enzyme that conveys resistance to -lactam antibiotics such as penicillins. For penicillin G, the KM for -lactamase is 13 μM. Which of the following plots represents inhibition of the reaction of -lactamase with penicillin G by clavulcanic acid, a competitive inhibitor? (Concent ...
Introduction_to_Enzymes (1)
... -explain why enzymes are necessary for life -state that enzymes are made of protein • Most people will -understand that an enzyme is a biological catalyst • Some people might -be able to write out word equations for enzyme reactions ...
... -explain why enzymes are necessary for life -state that enzymes are made of protein • Most people will -understand that an enzyme is a biological catalyst • Some people might -be able to write out word equations for enzyme reactions ...
week3-3
... It stabilizes the conformation that has a functional active site. B)- Allosteric inhibitors مثبطات: It stabilizes the conformation that lacks an active site. ...
... It stabilizes the conformation that has a functional active site. B)- Allosteric inhibitors مثبطات: It stabilizes the conformation that lacks an active site. ...
practice midterm answers
... 5) PMSF inactivates serine proteases by binding covalently to the catalytic site and this enzymeinhibitor bond cannot be cleaved by the enzyme. This is an example of A) irreversible inhibition B) competitive inhibition C) non-competitive inhibition D) mixed inhibition E) activation 6) Alpha helices ...
... 5) PMSF inactivates serine proteases by binding covalently to the catalytic site and this enzymeinhibitor bond cannot be cleaved by the enzyme. This is an example of A) irreversible inhibition B) competitive inhibition C) non-competitive inhibition D) mixed inhibition E) activation 6) Alpha helices ...
2. Enzymes
... *Allosteric Modulators - these bind away from active site but in doing so alter the shape of the active site. This can increase or decrease enzyme affinity for substrates. Covalent Modulators - bind covalently to enzyme away from the active site, change the shape, thus function of the enzyme. e.g., ...
... *Allosteric Modulators - these bind away from active site but in doing so alter the shape of the active site. This can increase or decrease enzyme affinity for substrates. Covalent Modulators - bind covalently to enzyme away from the active site, change the shape, thus function of the enzyme. e.g., ...
Catalysis by Enzymes
... and Allosteric Control • Concentration of thousands of different chemicals vary continuously in living organisms which requires regulation of enzyme activity. • Any process that starts or increase the activity of an enzyme is activation. • Any process that stops or slows the activity of an enzyme is ...
... and Allosteric Control • Concentration of thousands of different chemicals vary continuously in living organisms which requires regulation of enzyme activity. • Any process that starts or increase the activity of an enzyme is activation. • Any process that stops or slows the activity of an enzyme is ...
Enzymes - NVHSIntroBioPiper1
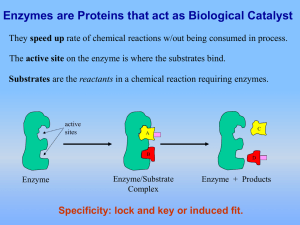
... on enzyme). While the enzyme and the substrate are joined, the enzyme catalyzes the reaction and converts the substrate to the product(s). ...
... on enzyme). While the enzyme and the substrate are joined, the enzyme catalyzes the reaction and converts the substrate to the product(s). ...
Enzymes and How They Work
... 2) Catalysts- chemicals which speed up a reaction without being consumed B. Properties of enzymes: 1) Speed up reactions 2) Can be used over and over, only needed in small amount 3) Decrease amount of energy needed to start a reaction (activation energy) 4) Specific to a substrate based on its shape ...
... 2) Catalysts- chemicals which speed up a reaction without being consumed B. Properties of enzymes: 1) Speed up reactions 2) Can be used over and over, only needed in small amount 3) Decrease amount of energy needed to start a reaction (activation energy) 4) Specific to a substrate based on its shape ...
Reading GuideMetabolismchapter6
... Another concept key to the topic of bacterial metabolism is enzymes and their function in metabolic pathways. Enzymes are proteins that lower the activation energy of a reaction. Without enzymes, these metabolic reactions would not occur. Enzymes have a specific site, called the active site where th ...
... Another concept key to the topic of bacterial metabolism is enzymes and their function in metabolic pathways. Enzymes are proteins that lower the activation energy of a reaction. Without enzymes, these metabolic reactions would not occur. Enzymes have a specific site, called the active site where th ...
How Did Life Begin? Unit Objectives Vocabulary: Miller
... o List the two components of cell theory and explain how they apply to the fossil record explored in unit 1 and the origin of life itself. o Explain the origin of organic molecules from inorganic matter. o Describe the Miller-Urey experiment, what it tested, and what the results indicate. o Describe ...
... o List the two components of cell theory and explain how they apply to the fossil record explored in unit 1 and the origin of life itself. o Explain the origin of organic molecules from inorganic matter. o Describe the Miller-Urey experiment, what it tested, and what the results indicate. o Describe ...
Lecture * 4 The Kinetics of Enzyme
... • Reversible inhibitors are termed competitive if their presence increases the value of Km but does not alter vmax The effect of such inhibitors can be countered or reversed by increasing the substrate concentration. • On the other hand, by rendering the enzyme or the enzyme-substrate complex inacti ...
... • Reversible inhibitors are termed competitive if their presence increases the value of Km but does not alter vmax The effect of such inhibitors can be countered or reversed by increasing the substrate concentration. • On the other hand, by rendering the enzyme or the enzyme-substrate complex inacti ...
Extension worksheet – Option C - Cambridge Resources for the IB
... Copy and complete this table to summarize the types of bond found in protein structure. Level of protein structure ...
... Copy and complete this table to summarize the types of bond found in protein structure. Level of protein structure ...
enzymes - SD57 Mail
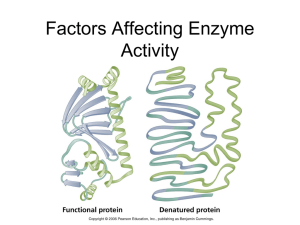
... • All enzymes have an optimal temperature • Up to this temperature, the reaction rate increases as temperature increases • Above this temperature, denaturation of the enzyme occurs (irreversible) ...
... • All enzymes have an optimal temperature • Up to this temperature, the reaction rate increases as temperature increases • Above this temperature, denaturation of the enzyme occurs (irreversible) ...
Enzymes - our Learning Areas
... catalysing reactions. • Many poisons work as enzyme inhibitors. • Also, unwanted enzyme activity may be controlled by inhibitors. • Sometimes reversible, sometimes not. Heavy metals (lead, mercury) prevent enzymes in cells of the nervous system from functioning. ...
... catalysing reactions. • Many poisons work as enzyme inhibitors. • Also, unwanted enzyme activity may be controlled by inhibitors. • Sometimes reversible, sometimes not. Heavy metals (lead, mercury) prevent enzymes in cells of the nervous system from functioning. ...
Enzymes I - eCurriculum
... Allosteric activators lock the enzyme in a conformation that has high affinity for the substrate Aspartate transcarbamylase ...
... Allosteric activators lock the enzyme in a conformation that has high affinity for the substrate Aspartate transcarbamylase ...
Reading GuideChapter6_Tues
... to function. Cofactors often improve the fit of the substrate with the active site of the enzyme (see Fig. 6.12). Some examples of cofactors are magnesium and zinc. Coenzymes are a special kind of organic cofactor that loosely bind molecules or carry electrons to help the reaction. Examples of coenz ...
... to function. Cofactors often improve the fit of the substrate with the active site of the enzyme (see Fig. 6.12). Some examples of cofactors are magnesium and zinc. Coenzymes are a special kind of organic cofactor that loosely bind molecules or carry electrons to help the reaction. Examples of coenz ...
Enzyme Inhibition
... CN- attach to the –SH groups in the enzyme This destroys the disulfide bridges and thus changing the tertiary structure of the enzyme Change in the shape results in the change in the active site thus the substrate cannot bind and cytochrome c oxidase is nonfuctional. ...
... CN- attach to the –SH groups in the enzyme This destroys the disulfide bridges and thus changing the tertiary structure of the enzyme Change in the shape results in the change in the active site thus the substrate cannot bind and cytochrome c oxidase is nonfuctional. ...
Enzymes
... -If pH of the substrate is higher or lower than optimum pH (highest enzyme activity) denaturation happens; enzyme becomes ineffective. -Different enzymes may have different optimum pH’s ...
... -If pH of the substrate is higher or lower than optimum pH (highest enzyme activity) denaturation happens; enzyme becomes ineffective. -Different enzymes may have different optimum pH’s ...
Lecture 20
... Digestive enzymes are produced as zymogens in one organ and transported to another, such as the pancreas, when needed activated by removing small peptide sections ...
... Digestive enzymes are produced as zymogens in one organ and transported to another, such as the pancreas, when needed activated by removing small peptide sections ...
Enzymes - Madison County Schools
... Equilibrium eventually reached b/c all the substrate is being broken down and adding more enzymes will not affect the reaction rate (Because those enzymes will have no substrate to break down). ...
... Equilibrium eventually reached b/c all the substrate is being broken down and adding more enzymes will not affect the reaction rate (Because those enzymes will have no substrate to break down). ...
Patrick_Chapter_4
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
Topic 3 Proteins as Drug Targets
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
01_Introduction. Structure, properties and biological functions
... •The enzyme cannot differentiate between the two compounds •When inhibitor binds, prevents the substrate from binding •Inhibitor can be released by increasing substrate concentration ...
... •The enzyme cannot differentiate between the two compounds •When inhibitor binds, prevents the substrate from binding •Inhibitor can be released by increasing substrate concentration ...
Enzymes - Pearland ISD
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.