* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Enzyme Inhibition
Nicotinamide adenine dinucleotide wikipedia , lookup
Cooperative binding wikipedia , lookup
Inositol-trisphosphate 3-kinase wikipedia , lookup
Restriction enzyme wikipedia , lookup
Alcohol dehydrogenase wikipedia , lookup
Transferase wikipedia , lookup
Lactoylglutathione lyase wikipedia , lookup
Beta-lactamase wikipedia , lookup
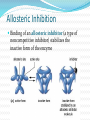
Part 2 INHIBITION ALLOSTERIC REGULATION FEEDBACK INHIBITION Remember… Enzymes catalyze (speed up) biological reactions The substrate (the reactants) must bind to the enzyme at the active site. Enzyme Animations: http://highered.mcgraw hill.com/sites/0072495855/student_view0/chapter2/animation__how_enzymes_wo rk.html http://www.youtube.com/watch?v=TLr7_2wnIXU http://www.youtube.com/watch?v=z8lG8X9ZvxQ&feature=related http://www.youtube.com/watch?v=CZD5xsOKres&feature=related http://www.youtube.com/watch?v=XTUm-75-PL4 Remember…. Enzymes are NOT reactants or products Enzymes are NOT used up in a reaction Enzymes may be used again over and over again (so long has they have not been denatured) Enzymes are specific to a particular substrate (or group of substrates) 1) Cells must control enzyme activity to coordinate cellular activities This can be done by: Restricting the production of a particular enzyme (enzymes are proteins, so your body can control how much you make of them) 1) Inhibiting the action of an enzyme This may involve a) competitive inhibition b) noncompetitive inhibition - including allosteric regulation c) feedback inhibition ENZYME INHBITION Sometimes enzyme function can be inhibited by other molecules (reducing the rate of enzyme-controlled reactions) These molecules are called INHIBITORS There are COMPETITIVE INHIBITORS and NON- COMPETITIVE INHIBITORS a) Competitive Inhibitors These are molecules that are similar in shape to the substrate They bind to the enzyme’s active site preventing the real substrate from binding The molecule “competes” with the substrate for the active site This can be overcome by increasing the substrate concentration Competitive Inhibitor Now the substrate cannot get to the active site because it is blocked by the inhibitor. enzyme substrate Ex: PRONTOSIL (competitive inhibitor) PRONTOSIL – is an antibacterial drug Bacteria require folic acid for replication of genetic material Prontosil binds to the enzyme that makes folic acid preventing other substrates from binding As a result, folic acid is not longer made and the bacterial cell dies Since animal cells don’t make folic acid themselves, they do not have this enzyme and so Prontosil has no effect on them b) Noncompetitive Inhibition A molecule binds to the enzyme at a location other than the active site This binding alters the shape of the enzyme, changing the shape of the active site The enzyme is now dysfunctional because the substrate now cannot bind to the active site. Adding more substrate will not affect the reaction because the active site is unavailable. Non competitive inhibitor substrate The shape of the active site has changed Therefore, the substrate no longer fits in enzyme Ex: Cytochrome C Oxidase (noncompetitive inhibition) Regular Function Speeds up the reduction of oxygen to water in cellular respiration Without it, the reaction will not occur fast enough and the organism will die because not enough energy is released. Ex: Cytochrome C Oxidase (noncompetitive inhibition) Inhibition CN- attach to the –SH groups in the enzyme This destroys the disulfide bridges and thus changing the tertiary structure of the enzyme Change in the shape results in the change in the active site thus the substrate cannot bind and cytochrome c oxidase is nonfuctional. Animations http://www.youtube.com/watch?v=PILzvT3spCQ Allosteric Regulation Some enzymes have receptor sites away from the active site called ALLOSTERIC SITES (These enzymes are usually proteins made of several subunits each with an active site) Substance that bind to the allosteric sites may inhibit or stimulate (increase) the enzyme activity. Allosteric Regulation - Activators Binding an ACTIVATOR to an allosteric site stabilizes the protein conformation This keeps all the active sites available for the substrates to bind to them. Allosteric Inhibition Binding of an allosteric inhibitor (a type of noncompetitive inhibitor) stabilizes the inactive form of the enzyme Allosteric Regulation The binding of an activator or an inhibitor affects the activity of all the active sites on the enzyme Animation: “ An example of allosteric activation of an enzyme” (Text video) c) Feedback Inhibition Method of controlling metabolic pathways Negative feedback inhibition is like a thermostat. When it is cold, the thermostat turns on the heater to produce heat. When it is too warm, the heat will cause the thermostat to turn off the heater Feedback Inhibition Cold Thermostat Heater Heat INHIBITS Heat has a negative effect on the thermostat; A build up of product inhibits the enzymes and the reaction Feedback Inhibition Many enzymatic pathways are regulated by feedback inhibition As the enzyme’s end product accumulates, it inhibits the enzyme by binding to the first enzyme in the pathway. This shuts down the entire sequence. Feedback Inhibition As the product is used up over time, the concentration of product decreases The inhibition product will detach from the enzyme allowing it to become active once again and produce product. When the product concentration gets too high again, the product will once again allosterically inhibit the enzyme Animations Feedback inhibition animation (text) http://highered.mcgraw- hill.com/sites/0072507470/student_view0/chapter2/a nimation__feedback_inhibition_of_biochemical_path ways.html