Enzymes
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
1 Name Chapter 3 Reading Guide Nucleic Acids, Proteins, and
... c. Explain the difference between your answer for the time of (A) and (B). Disulfide bridges are necessary for protein tertiary structure and must form before the enzyme active site can reappear, but there are other chemical interactions, such as hydrogen bonding and hydrophobic interactions, that o ...
... c. Explain the difference between your answer for the time of (A) and (B). Disulfide bridges are necessary for protein tertiary structure and must form before the enzyme active site can reappear, but there are other chemical interactions, such as hydrogen bonding and hydrophobic interactions, that o ...
Protein enzyme
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
... - strong enough to hold the substrate sufficiently long for the reaction to occur - weak enough to allow the product to depart Implies a fine balance Drug design - designing molecules with stronger binding interactions results in enzyme inhibitors which block the active site ...
Enzyme Activity with Graphs
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
... (1) An enzyme and a SUBSTRATE are in the same area. The substrate is the biological molecule that the enzyme will work on. (2) The enzyme grabs onto the substrate with a special area called the ACTIVE SITE. The active site is a specially shaped area of the enzyme that fits around the substrate. The ...
Case 13 Inhibition of Alcohol Dehydrogenase
... a. What are the KM and Vmax values for ADH in the absence of inhibitor? in the presence of the inhibitor? b. What type of inhibitor is N-1,5-dimethylhexylformamide? Explain. c. Calculate the values of " and/or "’, if they are significantly different from 1. What kind of inhibitor is N-1,5-dimethylh ...
... a. What are the KM and Vmax values for ADH in the absence of inhibitor? in the presence of the inhibitor? b. What type of inhibitor is N-1,5-dimethylhexylformamide? Explain. c. Calculate the values of " and/or "’, if they are significantly different from 1. What kind of inhibitor is N-1,5-dimethylh ...
Presentation
... Irreversible Enzyme Inhibition • Irreversible inhibitors form stable covalent bonds with the enzyme (e.g. alkylation or acylation of an active site side chain) • There are many naturally-occurring and synthetic irreversible inhibitors • These inhibitors can be used to identify the amino acid residu ...
... Irreversible Enzyme Inhibition • Irreversible inhibitors form stable covalent bonds with the enzyme (e.g. alkylation or acylation of an active site side chain) • There are many naturally-occurring and synthetic irreversible inhibitors • These inhibitors can be used to identify the amino acid residu ...
SI Worksheet 10 1. What does coupling reactions mean? The
... 10. Explain the mechanisms that underlie enzymes regarding the enzyme substrate theory? Is it known as ”Lock and Key” or “Induced Fit Hypothesis”? Substrates bind to an enzyme at a place known as the active site. Once the substrates have bound to the active site the enzyme changes shape and caudles ...
... 10. Explain the mechanisms that underlie enzymes regarding the enzyme substrate theory? Is it known as ”Lock and Key” or “Induced Fit Hypothesis”? Substrates bind to an enzyme at a place known as the active site. Once the substrates have bound to the active site the enzyme changes shape and caudles ...
practice midterm
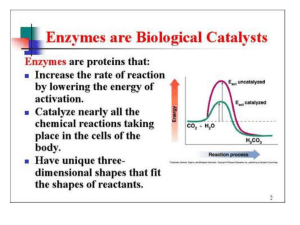
... D) increase the rate at which substrate is converted to product E) make the free energy change for the reaction more favorable 2) The number of substrate molecules converted to product in a given unit of time by a single enzyme molecule at saturation is referred to as the A) dissociation constant B) ...
... D) increase the rate at which substrate is converted to product E) make the free energy change for the reaction more favorable 2) The number of substrate molecules converted to product in a given unit of time by a single enzyme molecule at saturation is referred to as the A) dissociation constant B) ...
CATALYSIS OF BIOCHEMICAL REACTIONS
... It also places a partial charge on the substrate, making it react more easily with water (hydrolysis). ...
... It also places a partial charge on the substrate, making it react more easily with water (hydrolysis). ...
Ecotek Students Improve Protocol for the Enzyme Hydrolysis of Starch
... An enzyme is made up of a group of proteins that perform different biochemical functions. They serve as catalysts to speed up chemical reactions. An enzyme is formed by stringing together between 100 and 1,000 amino acids. The shape of an enzyme allows it to carry out specific chemical reactions. En ...
... An enzyme is made up of a group of proteins that perform different biochemical functions. They serve as catalysts to speed up chemical reactions. An enzyme is formed by stringing together between 100 and 1,000 amino acids. The shape of an enzyme allows it to carry out specific chemical reactions. En ...
Enzymes - Chemistry@Elmhurst
... Enzyme Inhibitors • Regulator or feedback - used to control a sequence of reactions - reaction product may block initial enzyme. ...
... Enzyme Inhibitors • Regulator or feedback - used to control a sequence of reactions - reaction product may block initial enzyme. ...
CHAPTER 1 - Portal UniMAP
... • IRREVERSIBLE ( like heavy metal)form a stable complex with enzyme and reduce enzyme activity • Such enzyme inhibition may be REVERSED only by using chelating agents such as EDTA and citrate. It is easily DISSOCIATED from the enzyme after binding. ...
... • IRREVERSIBLE ( like heavy metal)form a stable complex with enzyme and reduce enzyme activity • Such enzyme inhibition may be REVERSED only by using chelating agents such as EDTA and citrate. It is easily DISSOCIATED from the enzyme after binding. ...
Enzyme Introductory Lecture
... The activation energy for these substrates to bind together has been lowered by the enzyme. ...
... The activation energy for these substrates to bind together has been lowered by the enzyme. ...
enzymes - kristashunkwiler
... IV. Cofactors/Coenzymes • Non-protein molecules that help enzymes function. • Bind to active site to enhance enzymatic reactions or can bind to the substrate. • Cofactors may be inorganic metals such as zinc, iron, or copper. • Coenzymes are organic cofactors (e.g. vitamins) ...
... IV. Cofactors/Coenzymes • Non-protein molecules that help enzymes function. • Bind to active site to enhance enzymatic reactions or can bind to the substrate. • Cofactors may be inorganic metals such as zinc, iron, or copper. • Coenzymes are organic cofactors (e.g. vitamins) ...
PBHS AP Biology Lab 2
... attract H+ ions and the enzymes shape is disrupted As the pH goes up, the enzyme will lose H+ ions and again, the shaped is altered Optimum pH is in the neutral range At very low or high pH, the enzyme denatures (breaks down) ...
... attract H+ ions and the enzymes shape is disrupted As the pH goes up, the enzyme will lose H+ ions and again, the shaped is altered Optimum pH is in the neutral range At very low or high pH, the enzyme denatures (breaks down) ...
Enzymes
... enzymes is 7. Exceptions :Pepsin (gastric protease) Trypsin (intestinal protease) Pepsin works best at pH of 3 ...
... enzymes is 7. Exceptions :Pepsin (gastric protease) Trypsin (intestinal protease) Pepsin works best at pH of 3 ...
Biomolecule Test Review 2015
... Step 1: Substrate attaches to the enzyme at the active site (Like a lock and key it must be the right shape in order to fit…doesn’t fit doesn’t work) Step 2: Enzyme substrate complex formed – Reaction occurs Step 3 (What happens to the enzyme?): Products are released from the enzyme. Enzyme remains ...
... Step 1: Substrate attaches to the enzyme at the active site (Like a lock and key it must be the right shape in order to fit…doesn’t fit doesn’t work) Step 2: Enzyme substrate complex formed – Reaction occurs Step 3 (What happens to the enzyme?): Products are released from the enzyme. Enzyme remains ...
Enzymes
... Most important type of protein found in all living things Enzymes speed up chemical reactions in digestion of food, storage, synthesis of molecules and much more! ...
... Most important type of protein found in all living things Enzymes speed up chemical reactions in digestion of food, storage, synthesis of molecules and much more! ...
Test 2
... Competitive inhibitors bind reversibly to the active site of the enzyme. Uncompetitive inhibitors bind reversibly to some other site on the enzyme, and produce a change in the enzyme’s structure that in turn alters the enzyme’s kinetics. A suicide inhibitor is a compound that permanently binds to an ...
... Competitive inhibitors bind reversibly to the active site of the enzyme. Uncompetitive inhibitors bind reversibly to some other site on the enzyme, and produce a change in the enzyme’s structure that in turn alters the enzyme’s kinetics. A suicide inhibitor is a compound that permanently binds to an ...
Biomolecules Test Review -KEY
... Step 1: Substrate attaches to the enzyme at the active site (Like a lock and key it must be the right shape in order to fit…doesn’t fit doesn’t work) Step 2: Enzyme substrate complex formed – Reaction occurs Step 3 (What happens to the enzyme?): Products are released from the enzyme. Enzyme remains ...
... Step 1: Substrate attaches to the enzyme at the active site (Like a lock and key it must be the right shape in order to fit…doesn’t fit doesn’t work) Step 2: Enzyme substrate complex formed – Reaction occurs Step 3 (What happens to the enzyme?): Products are released from the enzyme. Enzyme remains ...
Amino Acids, Proteins, and Enzymes
... Sucrase has an optimum temperature of 37°C and an optimum pH of 6.2. Determine the effect of the following on its rate of reaction. 1) no change 2) increase 3) decrease A. Increasing the concentration of sucrose B. Changing the pH to 4 C. Running the reaction at 70°C ...
... Sucrase has an optimum temperature of 37°C and an optimum pH of 6.2. Determine the effect of the following on its rate of reaction. 1) no change 2) increase 3) decrease A. Increasing the concentration of sucrose B. Changing the pH to 4 C. Running the reaction at 70°C ...
ENZYMES PPT
... Some substances can inhibit enzyme function – Inhibitors Some substances can enhance enzyme function – activators ...
... Some substances can inhibit enzyme function – Inhibitors Some substances can enhance enzyme function – activators ...
1D17 – BMI201 Page 1 of 3 Code Questions Answers 1 Discuss the
... and are therefore water soluble, with more hydrophobic groups located on the interior of the protein, sheltered from the aqueous environment. In contrast, proteins that reside in the lipid environment of the cell membrane have mostly hydrophobic amino acids on their exterior surface and are not read ...
... and are therefore water soluble, with more hydrophobic groups located on the interior of the protein, sheltered from the aqueous environment. In contrast, proteins that reside in the lipid environment of the cell membrane have mostly hydrophobic amino acids on their exterior surface and are not read ...
Enzymes - Fairfield Public Schools
... diagram is the substrate? Explain. 4. At which step does the chemical reaction actually take place? 5. What chemical reaction is catalyzed by the enzyme? 6. How can you tell from the diagram that sucrase is not used up in the reaction? ...
... diagram is the substrate? Explain. 4. At which step does the chemical reaction actually take place? 5. What chemical reaction is catalyzed by the enzyme? 6. How can you tell from the diagram that sucrase is not used up in the reaction? ...
Bio-chemistry(Enzymes)
... Inhibitor binds at a site other than the active site on the enzyme surface. This binding impairs the enzyme function. The inhibitor has no structural resemblance with the substrate however there usually exists a strong affinity for the inhibitor to bind at the second site. Inhibitor does not interfe ...
... Inhibitor binds at a site other than the active site on the enzyme surface. This binding impairs the enzyme function. The inhibitor has no structural resemblance with the substrate however there usually exists a strong affinity for the inhibitor to bind at the second site. Inhibitor does not interfe ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.