GCSE worksheet on the development of the model of the atom
... All matter could be divided and sub-divided into smaller and smaller units, and eventually there would be a tiny particle that could not be divided any further - an atom. Atoms were different shapes and sizes. ...
... All matter could be divided and sub-divided into smaller and smaller units, and eventually there would be a tiny particle that could not be divided any further - an atom. Atoms were different shapes and sizes. ...
Nuclear ppt notes
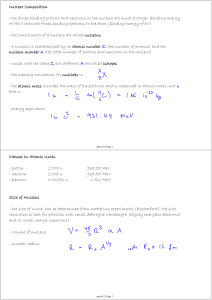
... The number of protons determines the properties of an atom (i.e. what type of atom it is) Carbon always has 6 protons, hydrogen always has 1 proton, oxygen always has 8 protons You change the # of protons, you change the type of atom Neutrons have no chemical effect on an atom’s properties ...
... The number of protons determines the properties of an atom (i.e. what type of atom it is) Carbon always has 6 protons, hydrogen always has 1 proton, oxygen always has 8 protons You change the # of protons, you change the type of atom Neutrons have no chemical effect on an atom’s properties ...
subatomic particle
... The smallest pieces of matter… • Nuclear physics and particle physics study the smallest known building blocks of the physical universe -and the interactions between them. • The focus is on single particles or small groups of particles, not the billions of atoms or molecules making up an entire pla ...
... The smallest pieces of matter… • Nuclear physics and particle physics study the smallest known building blocks of the physical universe -and the interactions between them. • The focus is on single particles or small groups of particles, not the billions of atoms or molecules making up an entire pla ...
AP Chem
... 7. How to calculate the amount of an isotope left after an integral number of half lives without a calculator. 8. Write nuclear equations. How to predict the relative stability of isotopes using mass number and atomic mass. 9. How to predict which type of decay an isotope will undergo using mass num ...
... 7. How to calculate the amount of an isotope left after an integral number of half lives without a calculator. 8. Write nuclear equations. How to predict the relative stability of isotopes using mass number and atomic mass. 9. How to predict which type of decay an isotope will undergo using mass num ...
nuclear chemistry - Wood County Schools
... Beta Decay: Medium-level radiation from the emission of beta particles (electrons). Positron Emission: Medium-level radiation from the emission of a positron, which is the same as an electron, only with a positive charge, converting a proton into a neutron. Electron Capture: When an atom takes in an ...
... Beta Decay: Medium-level radiation from the emission of beta particles (electrons). Positron Emission: Medium-level radiation from the emission of a positron, which is the same as an electron, only with a positive charge, converting a proton into a neutron. Electron Capture: When an atom takes in an ...
What`s common these things
... It has the fastest messengers on the short distance, the gluons (the “sticky” ones). They bind together quarks to form particles called “hadrons” like, for instance, protons and neutrons and indirectly nuclei. Exchanging gluons, quarks exchange their intrinsic color Electromagnetic interaction It af ...
... It has the fastest messengers on the short distance, the gluons (the “sticky” ones). They bind together quarks to form particles called “hadrons” like, for instance, protons and neutrons and indirectly nuclei. Exchanging gluons, quarks exchange their intrinsic color Electromagnetic interaction It af ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.