4.3 distinguishing among atoms
... 4.3 Distinguishing Among Atoms Review: Protons, neutrons and electrons are the sub particles of the atom Neutrons and protons are more massive than electron (determine the mass of atom) ...
... 4.3 Distinguishing Among Atoms Review: Protons, neutrons and electrons are the sub particles of the atom Neutrons and protons are more massive than electron (determine the mass of atom) ...
Name - Net Start Class
... 37) What is occurring in the nucleus during fission? It splits into two similar sized pieces. 38) What is the affect of nuclear absorption in a nuclear reactor? It makes the nuclear reaction slow down 39) What type of radiation is detected by a Geiger counter? radiation 40) What is the symbol for ...
... 37) What is occurring in the nucleus during fission? It splits into two similar sized pieces. 38) What is the affect of nuclear absorption in a nuclear reactor? It makes the nuclear reaction slow down 39) What type of radiation is detected by a Geiger counter? radiation 40) What is the symbol for ...
chapter 7 – cyu
... 3. Crookes put an iron cross in the middle. The cross blocked the rays coming from the cathode end. The shadow on the one end (anode) allowed him to see where the electrons were coming from. Crookes also had another experiment using a pinwheel in which electric currents, when switched on, would caus ...
... 3. Crookes put an iron cross in the middle. The cross blocked the rays coming from the cathode end. The shadow on the one end (anode) allowed him to see where the electrons were coming from. Crookes also had another experiment using a pinwheel in which electric currents, when switched on, would caus ...
Atomic Theory History Chem 11
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
teachers.sd43.bc.ca
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
strong force
... • This is explained by the quantum theory of the nucleus called the nuclear shell model • There are energy levels of nucleons inside a nucleus, ...
... • This is explained by the quantum theory of the nucleus called the nuclear shell model • There are energy levels of nucleons inside a nucleus, ...
Article 2: Key Concepts and Vocabulary
... charged particles A magnetic confinement fusion reactor features large and very strong magnets. The magnetic fields created by these magnets can be shaped into specific configurations that force all of the charged particles in the plasma to move within a specific region of the fusion reactor’s vacuu ...
... charged particles A magnetic confinement fusion reactor features large and very strong magnets. The magnetic fields created by these magnets can be shaped into specific configurations that force all of the charged particles in the plasma to move within a specific region of the fusion reactor’s vacuu ...
File - Mr. Gittermann
... with no charge and is located in the nucleus of the atom • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
... with no charge and is located in the nucleus of the atom • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
Review Notes - Biochemistry
... The goal of all atoms is to have a _STABLE_ outer energy level. The goal leads to bonding of atoms. 2 types of bonding: 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged ...
... The goal of all atoms is to have a _STABLE_ outer energy level. The goal leads to bonding of atoms. 2 types of bonding: 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged ...
Atomic (proton) number = is the number of protons found in the
... decay by emitting a gamma ray(s) and/or subatomic particles. Radionuclides may occur naturally, but can also be artificially produced. Isotopes = nuclides that have the same proton number but different mass numbers. Radioisotope = radioactive species of a given element Isomers = two nuclides that di ...
... decay by emitting a gamma ray(s) and/or subatomic particles. Radionuclides may occur naturally, but can also be artificially produced. Isotopes = nuclides that have the same proton number but different mass numbers. Radioisotope = radioactive species of a given element Isomers = two nuclides that di ...
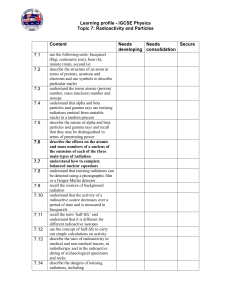
What do I know about……
... minute (min), second (s) describe the structure of an atom in terms of protons, neutrons and electrons and use symbols to describe particular nuclei understand the terms atomic (proton) number, mass (nucleon) number and isotope understand that alpha and beta particles and gamma rays are ionising rad ...
... minute (min), second (s) describe the structure of an atom in terms of protons, neutrons and electrons and use symbols to describe particular nuclei understand the terms atomic (proton) number, mass (nucleon) number and isotope understand that alpha and beta particles and gamma rays are ionising rad ...
National Science Week Event with Girlguiding Worcestershire
... Given the level of the question, I assumed an age of about 14. So I went straight in with: “Have you come across the model of the atom with the nucleus in the middle and the electrons whizzing round it?”. “Ummmm… no I don’t think so…”, came the reply. At this point, the adult next to her said “No, s ...
... Given the level of the question, I assumed an age of about 14. So I went straight in with: “Have you come across the model of the atom with the nucleus in the middle and the electrons whizzing round it?”. “Ummmm… no I don’t think so…”, came the reply. At this point, the adult next to her said “No, s ...
Nuclear Chemistry
... mass of all the atoms of that element, taking into account the varying numbers of neutrons. Neutrons do not play a role in determining the identity of an atom. ...
... mass of all the atoms of that element, taking into account the varying numbers of neutrons. Neutrons do not play a role in determining the identity of an atom. ...
THINGSYOUNEEDTOKNOW-modern
... The sum of all charge is conserved in the universe. Conservation means all BEFORE = all AFTER Know the RRT Standard Model section Protons are baryons made of u-u-d quarks Neutrons are baryons made of u-d-d quarks All quarks and leptons have antiparticle counterparts. All things the same except oppos ...
... The sum of all charge is conserved in the universe. Conservation means all BEFORE = all AFTER Know the RRT Standard Model section Protons are baryons made of u-u-d quarks Neutrons are baryons made of u-d-d quarks All quarks and leptons have antiparticle counterparts. All things the same except oppos ...
Chemistry Study Guide
... 1. Using the periodic table, how many protons, neutrons, and electrons are in an atom of cobalt? P = 27 E = 27 N = 32 *Electrons =protons, protons = atomic number, neutrons = atomic mass - protons 2. Which particles are located in the nucleus of the atom? Protons & neutrons 3. What is a molecule? Be ...
... 1. Using the periodic table, how many protons, neutrons, and electrons are in an atom of cobalt? P = 27 E = 27 N = 32 *Electrons =protons, protons = atomic number, neutrons = atomic mass - protons 2. Which particles are located in the nucleus of the atom? Protons & neutrons 3. What is a molecule? Be ...
solutions
... Problem 3. Nobel laureate Richard Feynman once said that if two persons stood at arm’s length from each other and each person had p = 1% more electrons than protons, the force of repulsion between them would be enough to lift a “weight” equal to that of the entire Earth. Carry out an order of magnit ...
... Problem 3. Nobel laureate Richard Feynman once said that if two persons stood at arm’s length from each other and each person had p = 1% more electrons than protons, the force of repulsion between them would be enough to lift a “weight” equal to that of the entire Earth. Carry out an order of magnit ...
Answers - Manhattan Press
... Worksheet 3 Rutherford – father of nuclear physics Time to Think (p. 17) ...
... Worksheet 3 Rutherford – father of nuclear physics Time to Think (p. 17) ...
Chapter 8 Study Guide
... a. Chemists discovered that if two or more different compounds are composed of the same elements, the ratio of the masses of the second element is always a ratio of small whole numbers. This example illustrates the law of multiple proportions ...
... a. Chemists discovered that if two or more different compounds are composed of the same elements, the ratio of the masses of the second element is always a ratio of small whole numbers. This example illustrates the law of multiple proportions ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.