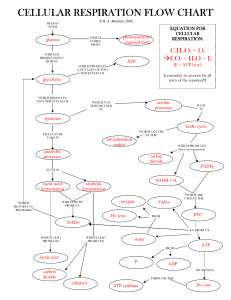
6.1 Cellular respiration
... of cellular respiration. Glucose metabolism Cellular respiration = glucose oxidation Glucose 1 oxygen → carbon dioxide 1 water 1 energy (ATP) This reaction does not occur in one simple reaction, but involves over each controlled by specific enzymes. ...
... of cellular respiration. Glucose metabolism Cellular respiration = glucose oxidation Glucose 1 oxygen → carbon dioxide 1 water 1 energy (ATP) This reaction does not occur in one simple reaction, but involves over each controlled by specific enzymes. ...
College Prep Cellular Respiration Notes: H.B.3A.4 Harvesting
... • A chemical reaction in which there is the transfer of one or more electrons from one reactant to another. • Oxidation is the loss of electrons • Reduction is the gain of electrons. • Because the electron transfer requires a donor and an acceptor, oxidation and reduction always go together. What Ca ...
... • A chemical reaction in which there is the transfer of one or more electrons from one reactant to another. • Oxidation is the loss of electrons • Reduction is the gain of electrons. • Because the electron transfer requires a donor and an acceptor, oxidation and reduction always go together. What Ca ...
chapter_6_mod_2009
... Since prokaryotes have no mitochondria, it all occurs in the cytoplasm. Make 2 more ATP because there is a cost to the eukaryotic cell of getting the electrons into the mitochondrion ...
... Since prokaryotes have no mitochondria, it all occurs in the cytoplasm. Make 2 more ATP because there is a cost to the eukaryotic cell of getting the electrons into the mitochondrion ...
Cell Respiration copy
... Acid is produced n Sore muscles after a workout n How bacteria makes yogurt ...
... Acid is produced n Sore muscles after a workout n How bacteria makes yogurt ...
File
... ATP is continuously made at the same time as it is being used up, so there is no need for humans to have a vast store of ATP Phosphorylation is an enzyme controlled process by which a phosphate group is added to a molecule Phosphorylation also occurs when the phosphate and energy are transferred fro ...
... ATP is continuously made at the same time as it is being used up, so there is no need for humans to have a vast store of ATP Phosphorylation is an enzyme controlled process by which a phosphate group is added to a molecule Phosphorylation also occurs when the phosphate and energy are transferred fro ...
bio II ch 8 brookings guided pp
... Most of the ___________________is produced in the Krebs cycle. Not much immediate energy (only 1 ATP). ...
... Most of the ___________________is produced in the Krebs cycle. Not much immediate energy (only 1 ATP). ...
3 " ‡ ‡ ‡ ‡ ‡ ‡ ‡ ‡ ‡ ‡ ‡ - 1 - G 2 ¢ 2 2 – 1. Biological catalysts are (A
... (D) leucine and isoleucine 9. At pH below 7, arginine has a net charge of (A) +2 (B) +1 ...
... (D) leucine and isoleucine 9. At pH below 7, arginine has a net charge of (A) +2 (B) +1 ...
chapter9sganswers
... IMPORTANT: H+ ions cannot move back through the proteins of the ETC (these are a one way street, from matrix to intermembrane space only) or through the phospholipid bilayer of the inner membrane of the mitochondria (because H+ are charged, polar molecules and the membrane in mainly nonpolar (see ch ...
... IMPORTANT: H+ ions cannot move back through the proteins of the ETC (these are a one way street, from matrix to intermembrane space only) or through the phospholipid bilayer of the inner membrane of the mitochondria (because H+ are charged, polar molecules and the membrane in mainly nonpolar (see ch ...
File
... • Electron Transport – Electrons move through the inner membrane via a series of carriers of decreasing redox potential. – Electrons associated with either NADH or FADH2 are transferred through specific electron carriers that make up the electron transport chain. ...
... • Electron Transport – Electrons move through the inner membrane via a series of carriers of decreasing redox potential. – Electrons associated with either NADH or FADH2 are transferred through specific electron carriers that make up the electron transport chain. ...
2. What are the main properties that fats, proteins, and
... Like all proteins, enzymes are long, linear chains of amino acids that fold to produce a three-dimensional product. Each unique amino acid sequence produces a specific structure, which has unique properties. 26. How many ways do you know that an enzymatic activity can be regulated/controlled/inhibit ...
... Like all proteins, enzymes are long, linear chains of amino acids that fold to produce a three-dimensional product. Each unique amino acid sequence produces a specific structure, which has unique properties. 26. How many ways do you know that an enzymatic activity can be regulated/controlled/inhibit ...
AP Biology Chap 9 Reading Guide Cellular Respiration
... 17. Notice that glycolysis occurs in the ________________________ of the cell. What is the relationship concerning glycolysis and oxygen? Concept 9.3 The citric acid cycle completes the energy-yielding oxidation of organic molecules 18. To enter the citric acid cycle, pyruvate must enter the mitocho ...
... 17. Notice that glycolysis occurs in the ________________________ of the cell. What is the relationship concerning glycolysis and oxygen? Concept 9.3 The citric acid cycle completes the energy-yielding oxidation of organic molecules 18. To enter the citric acid cycle, pyruvate must enter the mitocho ...
Slide 1 - MisterSyracuse.com
... 31. The steps of mitosis are much like a well-choreographed dance; they must go in just the right order, or the whole thing falls apart. Most of the time, mitosis goes very smoothly. However, mistakes can be made. One such mistake has been recently reported in a paper from 2005. The scientists who d ...
... 31. The steps of mitosis are much like a well-choreographed dance; they must go in just the right order, or the whole thing falls apart. Most of the time, mitosis goes very smoothly. However, mistakes can be made. One such mistake has been recently reported in a paper from 2005. The scientists who d ...
Chapter Six – Chemistry in Biology – Study Guide for End of Chapter
... Energy is either released or used Activation energy – amount needed to get a reaction going Catalyst / enzyme – special chemicals which by their presence lower the activation energy and speed up reactions without taking part in the reactions – act like a lock / key mechanism ALWAYS the same ...
... Energy is either released or used Activation energy – amount needed to get a reaction going Catalyst / enzyme – special chemicals which by their presence lower the activation energy and speed up reactions without taking part in the reactions – act like a lock / key mechanism ALWAYS the same ...
Assessment
... _____ 19. Two products of the Krebs cycle are a. H2O and CO2. b. ATP and O2. c. ADP and H2O _____ 20. What provides the electron transport chain in cellular respiration with the energy it needs to function? a. glycolysis b. chlorophyll c. Krebs cycle _____ 21. Which of the following statements best ...
... _____ 19. Two products of the Krebs cycle are a. H2O and CO2. b. ATP and O2. c. ADP and H2O _____ 20. What provides the electron transport chain in cellular respiration with the energy it needs to function? a. glycolysis b. chlorophyll c. Krebs cycle _____ 21. Which of the following statements best ...
Review Packet CORRECT
... c. Describe the movement of electrons in OP They move through the electron transport chain and are DE energized. d. Describe the movement of H+ ions (protons) in OP They are pumped from the matrix (low concentration) to the intermembrane space (high concentration) ...
... c. Describe the movement of electrons in OP They move through the electron transport chain and are DE energized. d. Describe the movement of H+ ions (protons) in OP They are pumped from the matrix (low concentration) to the intermembrane space (high concentration) ...
Respiration
... When ___________enters the ETC, it becomes the final electron acceptor of the Hydrogen ions and creates________. As the hydrogen ions come back across the membrane, ADP is converted into ______ ...
... When ___________enters the ETC, it becomes the final electron acceptor of the Hydrogen ions and creates________. As the hydrogen ions come back across the membrane, ADP is converted into ______ ...
FREE Sample Here
... hydrogen ions pass from the mitochondrial matrix to the intermembrane space, activating ATP synthase. b. hydrogen ions pass from the intermembrane space to the mitochondrial matrix, activating ATP synthase. c. water passes from the mitochondrial matrix to the intermembrane space, activating ATP synt ...
... hydrogen ions pass from the mitochondrial matrix to the intermembrane space, activating ATP synthase. b. hydrogen ions pass from the intermembrane space to the mitochondrial matrix, activating ATP synthase. c. water passes from the mitochondrial matrix to the intermembrane space, activating ATP synt ...
atoms - St. Clair Schools
... • Anything that takes up space and has mass • Made up of particles called atoms ...
... • Anything that takes up space and has mass • Made up of particles called atoms ...
Glycolysis, Krebs cycle and Cytochrome chain
... During this cycle 1 molecule of ATP is produced, 3x NADH and 1xFADH2 and also 2x CO2. The hydrogens and e- from the Co-factor molecules NADH and FADH2 now enter the cytochrome chain.(stage 3) The carbon atoms of the glucose molecules are now completely oxidised some of the energy of glucose has been ...
... During this cycle 1 molecule of ATP is produced, 3x NADH and 1xFADH2 and also 2x CO2. The hydrogens and e- from the Co-factor molecules NADH and FADH2 now enter the cytochrome chain.(stage 3) The carbon atoms of the glucose molecules are now completely oxidised some of the energy of glucose has been ...
1 Chapter 5 Microbial Metabolism 2
... Pyruvic acid (from glycolysis) is oxidized and decarboxylated The Krebs Cycle Oxidation of acetyl CoA produces NADH and FADH2 The Electron Transport Chain A series of carrier molecules that are, in turn, oxidized and reduced as electrons are passed down the chain Energy released can be used to produ ...
... Pyruvic acid (from glycolysis) is oxidized and decarboxylated The Krebs Cycle Oxidation of acetyl CoA produces NADH and FADH2 The Electron Transport Chain A series of carrier molecules that are, in turn, oxidized and reduced as electrons are passed down the chain Energy released can be used to produ ...
Substrate Level Phosphorylation Substrate level phosphorylation
... •Catabolic reactions: reactants act as “fuels,” broken down with the help of enzymes •Fermentation: sugar degradation without oxygen (anaerobic) •Cellular Respiration: most efficient and prevalent means of ...
... •Catabolic reactions: reactants act as “fuels,” broken down with the help of enzymes •Fermentation: sugar degradation without oxygen (anaerobic) •Cellular Respiration: most efficient and prevalent means of ...
Chapter 8 - University of South Alabama
... ATPs for a grand total of 36 ATPs per glucose molecule. ...
... ATPs for a grand total of 36 ATPs per glucose molecule. ...
No Slide Title
... 6) Reaction 4: Isocitrate and NAD+ react to form the energy carrier and oxalosuccinate. 7) Reaction 5: Oxalosuccinate loses a molecule of CO2, forming ketoglutarate. 8) Reaction 6: Ketoglutarate hooks up with Coenzyme A to form succinyl CoA. This process releases 2 electrons and H to form NADH. 9) I ...
... 6) Reaction 4: Isocitrate and NAD+ react to form the energy carrier and oxalosuccinate. 7) Reaction 5: Oxalosuccinate loses a molecule of CO2, forming ketoglutarate. 8) Reaction 6: Ketoglutarate hooks up with Coenzyme A to form succinyl CoA. This process releases 2 electrons and H to form NADH. 9) I ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.