Analysis and simulation of metabolic networks: Application to HEPG2
... Systems Toxicology integrates the methodologies of conventional toxicology, of high throughput molecular genomics, proteomics, metabonomics with bioinfomatics to analyse and predict the effects of environmental stressors or toxicants from chemical exposure to adverse effects. The final objective of ...
... Systems Toxicology integrates the methodologies of conventional toxicology, of high throughput molecular genomics, proteomics, metabonomics with bioinfomatics to analyse and predict the effects of environmental stressors or toxicants from chemical exposure to adverse effects. The final objective of ...
Introduction
... 1. The general characteristics and biological functions of proteins and peptides. 2. Amino acid composition of proteins and peptides: structure, classification and physical and chemical properties of amino acids. 3. The formation of peptide bonds. Levels of protein structure. Chemical bonds in prote ...
... 1. The general characteristics and biological functions of proteins and peptides. 2. Amino acid composition of proteins and peptides: structure, classification and physical and chemical properties of amino acids. 3. The formation of peptide bonds. Levels of protein structure. Chemical bonds in prote ...
Principles of BIOCHEMISTRY
... mitochondria into the blood and are transported to peripheral tissues. Ketone bodies are important molecules in energy metabolism. Heart muscle and the renal cortex use acetoacetate in preference to glucose in physiological conditions. The brain adapts to the utilization of acetoacetate during starv ...
... mitochondria into the blood and are transported to peripheral tissues. Ketone bodies are important molecules in energy metabolism. Heart muscle and the renal cortex use acetoacetate in preference to glucose in physiological conditions. The brain adapts to the utilization of acetoacetate during starv ...
Sites of enzyme activity along the nephron
... have previously been used as marker enzymes for glycolysis [10] demonstrated a distribution pattern which was quite inverse to thai of hexokinase and pyruvate kinase. G lyceraldehyde-3-phosphate dehydrogenase activities decreased from the proximal to the distal convoluted tubule. Lactate dehydrogena ...
... have previously been used as marker enzymes for glycolysis [10] demonstrated a distribution pattern which was quite inverse to thai of hexokinase and pyruvate kinase. G lyceraldehyde-3-phosphate dehydrogenase activities decreased from the proximal to the distal convoluted tubule. Lactate dehydrogena ...
Activity of ribosomes and tmRNA of Streptomyces aureofaciens
... producer Streptomyces aureofaciens under temperature shift up and down and possible role of transtranslation system. Tetracycline inhibits protein synthesis by interfering with the binding of aminoacyltRNA to the A-site of ribosome [14]. There are at least two functionally important binding sites fo ...
... producer Streptomyces aureofaciens under temperature shift up and down and possible role of transtranslation system. Tetracycline inhibits protein synthesis by interfering with the binding of aminoacyltRNA to the A-site of ribosome [14]. There are at least two functionally important binding sites fo ...
Enzyme Mechanisms - Illinois Institute of Technology
... d[B]/dt = v = k[A] where v is the velocity of the reaction and k is a constant known as the forward rate constant. Here, since [A] has dimensions of concentration and d[B]/dt has dimensions of concentration / time, the dimensions of k will be those of inverse time, e.g. sec-1. ...
... d[B]/dt = v = k[A] where v is the velocity of the reaction and k is a constant known as the forward rate constant. Here, since [A] has dimensions of concentration and d[B]/dt has dimensions of concentration / time, the dimensions of k will be those of inverse time, e.g. sec-1. ...
Stoking the Brightest Fires of Life Among Vertebrates
... These estimated flux rates can be compared with enzymatic flux capacities or Vmax values to yield further insights. However, further discussion should be preceded by a brief outline of some basic principles. Vmax values, measured in vitro under optimal conditions, equal [E] × kcat , where [E] is enz ...
... These estimated flux rates can be compared with enzymatic flux capacities or Vmax values to yield further insights. However, further discussion should be preceded by a brief outline of some basic principles. Vmax values, measured in vitro under optimal conditions, equal [E] × kcat , where [E] is enz ...
Electron transfer from aromatic amino acids to guanine and adenine
... strand breaks, protein–DNA cross-links etc.1–10 As DNA is an efficient carrier of hole11,12 and excess electron charges,13–16 the generated charge may migrate through the p-stack long distances away from the site of its initial formation and then initiate a DNA lesion. In living cells, this ability o ...
... strand breaks, protein–DNA cross-links etc.1–10 As DNA is an efficient carrier of hole11,12 and excess electron charges,13–16 the generated charge may migrate through the p-stack long distances away from the site of its initial formation and then initiate a DNA lesion. In living cells, this ability o ...
tetrahedron report number 124 suicide substrates
... Only after binding to the target enzyme and after the enzyme begins catalysis is the reactive chemical grouping uncovered. The particular chemical reaction sequence of the given enzyme is required to unravel the inactivator. This activation occurs in a precise microenvironment only, the active site ...
... Only after binding to the target enzyme and after the enzyme begins catalysis is the reactive chemical grouping uncovered. The particular chemical reaction sequence of the given enzyme is required to unravel the inactivator. This activation occurs in a precise microenvironment only, the active site ...
06_Metabolism of lipid
... • FA synthesis and degradation occur by two completely separate pathways • When glucose is plentiful, large amounts of acetyl CoA are produced by glycolysis and can be used for fatty acid synthesis ...
... • FA synthesis and degradation occur by two completely separate pathways • When glucose is plentiful, large amounts of acetyl CoA are produced by glycolysis and can be used for fatty acid synthesis ...
Carbamoyl phosphate synthetase - Department of Biochemistry
... with a thioester intermediate. Additional mutations of residues His353 and His312 have implicated these residues in the activation of the thiolate anion and binding of glutamine, respectively [22]. T h e working model for the hydrolysis of glutamine and generation of ammonia is presented in Figure 3 ...
... with a thioester intermediate. Additional mutations of residues His353 and His312 have implicated these residues in the activation of the thiolate anion and binding of glutamine, respectively [22]. T h e working model for the hydrolysis of glutamine and generation of ammonia is presented in Figure 3 ...
Probing Allosteric Binding Sites of the Maize
... molecules are bound in two of the four presumptive active sites, consistent with the notion that the protein functions as a dimer of dimers. On the other hand, one of the sulfate ions originally found in site 3 is lost when ATP is bound, despite the large distance between their respective binding si ...
... molecules are bound in two of the four presumptive active sites, consistent with the notion that the protein functions as a dimer of dimers. On the other hand, one of the sulfate ions originally found in site 3 is lost when ATP is bound, despite the large distance between their respective binding si ...
Hexose Monophosphate Shunt (HMP Shunt)
... HMP shunt (PPP) is less active in skeletal muscle & non-lactating mammary glands Site:- ...
... HMP shunt (PPP) is less active in skeletal muscle & non-lactating mammary glands Site:- ...
STRUCTURAL AND FUNCTIONAL STUDIES OF PYRIDOXINE 5’-PHOSPHATE SYNTHASE E. COLI Doctoral Thesis
... pyridoxine (PN), pyridoxal (PL), pyridoxamine (PM), PLP, and PMP are generated from PNP and interconverted into each other in the so-called salvage pathway by the action of the ATP-dependent kinase PdxK, various transaminases and the FMN-dependent oxidase PdxH (Dempsey, 1987; Hill and Spenser, 1986; ...
... pyridoxine (PN), pyridoxal (PL), pyridoxamine (PM), PLP, and PMP are generated from PNP and interconverted into each other in the so-called salvage pathway by the action of the ATP-dependent kinase PdxK, various transaminases and the FMN-dependent oxidase PdxH (Dempsey, 1987; Hill and Spenser, 1986; ...
Identification of Aspartate- 184 as an Essential Residue in the
... profile of the tryptic peptides and the accompanying radioactivity profiles. The inhibitor peptide and MgATP almost completely blocked the labeling of Glu-230. Mg2+ binds weakly to the C subunit, and there is some evidence suggesting that a carboxyl group may be involved as a chelator of a metal. Fo ...
... profile of the tryptic peptides and the accompanying radioactivity profiles. The inhibitor peptide and MgATP almost completely blocked the labeling of Glu-230. Mg2+ binds weakly to the C subunit, and there is some evidence suggesting that a carboxyl group may be involved as a chelator of a metal. Fo ...
The monocarboxylate transporter family
... about 2 nM (19). This very high-affinity enabled us to determine the number of molecules of MCT1 per erythrocyte (80,000) and the turnover number (kcat) of the transporter (12 s21 at 68C). We also characterized the inhibition by AR-C155858 of different MCT isoforms expressed in Xenopus oocytes and fo ...
... about 2 nM (19). This very high-affinity enabled us to determine the number of molecules of MCT1 per erythrocyte (80,000) and the turnover number (kcat) of the transporter (12 s21 at 68C). We also characterized the inhibition by AR-C155858 of different MCT isoforms expressed in Xenopus oocytes and fo ...
Acute nutritional ketosis: implications for exercise performance and metabolism Open Access
... Randle and colleagues described the glucose-free fatty acid (FFA) cycle in 1963, suggesting an overall substrate hierarchy dominated by fatty acid selection in preference to carbohydrate for oxidative phosphorylation [38]. The capacity of the mitochondria to alter its preferential fuel selection was ...
... Randle and colleagues described the glucose-free fatty acid (FFA) cycle in 1963, suggesting an overall substrate hierarchy dominated by fatty acid selection in preference to carbohydrate for oxidative phosphorylation [38]. The capacity of the mitochondria to alter its preferential fuel selection was ...
The Plasma Membrane - Beck-Shop
... instances lipoproteins extend outward from the plasma membrane and consist of carbohydrate polymers that are covalently linked to protein in the plasma membrane. The membrane is not rigid with the lipids covalently linked to other lipids or to proteins but forms a closed structure as a result of com ...
... instances lipoproteins extend outward from the plasma membrane and consist of carbohydrate polymers that are covalently linked to protein in the plasma membrane. The membrane is not rigid with the lipids covalently linked to other lipids or to proteins but forms a closed structure as a result of com ...
High resolution crystal structures of unliganded
... The amino acid sequences of the ACBPs of known structure, including the sequence of the human ACBP (hLACBP), are listed in Figure 1. All known structures have the same four-helix bundle fold (helices A1, A2, A3, and A4), with a 13-residue loop insertion between the parallel helix-pair of A2 and A3. ...
... The amino acid sequences of the ACBPs of known structure, including the sequence of the human ACBP (hLACBP), are listed in Figure 1. All known structures have the same four-helix bundle fold (helices A1, A2, A3, and A4), with a 13-residue loop insertion between the parallel helix-pair of A2 and A3. ...
Enzymes:The Catalysts of Life I
... If you stop to think about it, you are already familiar with many reactions that are thermodynamically feasible yet do not occur to any appreciable extent. An obvious example from Chapter 5 is the oxidation of glucose (see Reaction 5-1). This reaction (or series of reactions, really) is highly exerg ...
... If you stop to think about it, you are already familiar with many reactions that are thermodynamically feasible yet do not occur to any appreciable extent. An obvious example from Chapter 5 is the oxidation of glucose (see Reaction 5-1). This reaction (or series of reactions, really) is highly exerg ...
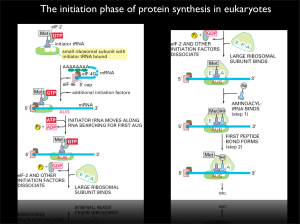
The initiation phase of protein synthesis in eukaryotes
... protein kinase-1(MNK1; also called MAP kinase signal-integrating kinase). MNK1 was identified independently by two groups as a substrate for ERK1 and ...
... protein kinase-1(MNK1; also called MAP kinase signal-integrating kinase). MNK1 was identified independently by two groups as a substrate for ERK1 and ...
Chapter 5
... What are the principal products of the Krebs cycle? 5-13 How do carrier molecules function in the electron transport chain? 5-14 Compare the energy yield (ATP) of aerobic and anaerobic respiration. 5-15 List four compounds that can be made from pyruvic acid by an organism that uses fermentat ...
... What are the principal products of the Krebs cycle? 5-13 How do carrier molecules function in the electron transport chain? 5-14 Compare the energy yield (ATP) of aerobic and anaerobic respiration. 5-15 List four compounds that can be made from pyruvic acid by an organism that uses fermentat ...
Chapter 5
... What are the principal products of the Krebs cycle? 5-13 How do carrier molecules function in the electron transport chain? 5-14 Compare the energy yield (ATP) of aerobic and anaerobic respiration. 5-15 List four compounds that can be made from pyruvic acid by an organism that uses fermentat ...
... What are the principal products of the Krebs cycle? 5-13 How do carrier molecules function in the electron transport chain? 5-14 Compare the energy yield (ATP) of aerobic and anaerobic respiration. 5-15 List four compounds that can be made from pyruvic acid by an organism that uses fermentat ...
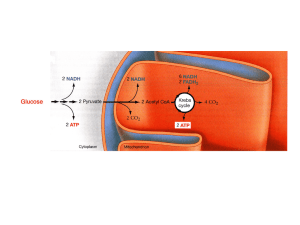
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.