Module 1. General principles of metabolism. Metabolism of
... 80. Conserved serine, histidine and aspartate residues are present in the catalytic center of all serine proteases. Which of the following describes the role of the histidine residue in the mechanism of this reaction? A. * Covalent binding of acyl groups B. Hydrophobic stabilization of the substrate ...
... 80. Conserved serine, histidine and aspartate residues are present in the catalytic center of all serine proteases. Which of the following describes the role of the histidine residue in the mechanism of this reaction? A. * Covalent binding of acyl groups B. Hydrophobic stabilization of the substrate ...
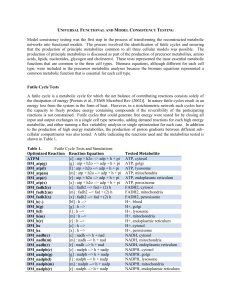
CBS (EC 4.2.1.22). The rate equation for the CBS reaction
... Concentrations of ATP, adenosine, betaine, dimethylglycine, glycine, NADPH, and serine, as well as a total concentration of all intracellular folates (F0) are assumed to be constant. In this way, either there is no dependence of reaction rates on these metabolites, or they are included in the releva ...
... Concentrations of ATP, adenosine, betaine, dimethylglycine, glycine, NADPH, and serine, as well as a total concentration of all intracellular folates (F0) are assumed to be constant. In this way, either there is no dependence of reaction rates on these metabolites, or they are included in the releva ...
L-Carnitine in human metabolism
... swollen mitochondria seen with electron microscopy (C, D; arrows). Immunohistochemistry with antibodies directed against aldehyde 4-hydroxy-2-nonenal 4HNE (E , F) and sarcoendoplasmic reticulum calcium ATPase SERCA-SO 3 shows increased staining (F, H) compared to normal controls ( E, G) ...
... swollen mitochondria seen with electron microscopy (C, D; arrows). Immunohistochemistry with antibodies directed against aldehyde 4-hydroxy-2-nonenal 4HNE (E , F) and sarcoendoplasmic reticulum calcium ATPase SERCA-SO 3 shows increased staining (F, H) compared to normal controls ( E, G) ...
Universitat Autònoma SEPARACIÓ DE COMPOSTOS ANIÒNICS I NEUTRES AMB
... In the case of using the amino acids as analyte, the affinity of its anionic form for the metallic center (Pd(II)) and the lower pH in the receiving phase that changes the speciation of the amino acid leading to its release from the LM phase is the power that maintains the transport. This system was ...
... In the case of using the amino acids as analyte, the affinity of its anionic form for the metallic center (Pd(II)) and the lower pH in the receiving phase that changes the speciation of the amino acid leading to its release from the LM phase is the power that maintains the transport. This system was ...
Universal Functional and Model Consistency Testing
... Validation of the myocyte network included simulating the ATP production from one mole of octadecenoate (C18:1), palmitate (C16:0) and pentadecanoate (C15:0) (Table 2). To demonstrate how each of these fatty acids could be catabolized to produce energy, the influx of all other carbon sources includ ...
... Validation of the myocyte network included simulating the ATP production from one mole of octadecenoate (C18:1), palmitate (C16:0) and pentadecanoate (C15:0) (Table 2). To demonstrate how each of these fatty acids could be catabolized to produce energy, the influx of all other carbon sources includ ...
Hydrogen Bonds
... A superscript plus or minus sign following the symbol of an element indicates an ion. A single plus sign indicates a cation with a charge of 1. (The original atom has lost one electron.) A single minus sign indicates an anion with a charge of 1. (The original atom has gained one electron.) If more ...
... A superscript plus or minus sign following the symbol of an element indicates an ion. A single plus sign indicates a cation with a charge of 1. (The original atom has lost one electron.) A single minus sign indicates an anion with a charge of 1. (The original atom has gained one electron.) If more ...
Electrolytes and metabolic disorder.
... Normal energy supply for the heart: fatty acid oxidation, 10-40% energy derived from pyruvate (from glycolysis or conversion of lactate) FAs have higher yields of ATP/molecule, ATP yield/O2 molecule is 5%-10% better with lactate and glucose Exercise, inotropes and fast pacing: lactate uptake by myoc ...
... Normal energy supply for the heart: fatty acid oxidation, 10-40% energy derived from pyruvate (from glycolysis or conversion of lactate) FAs have higher yields of ATP/molecule, ATP yield/O2 molecule is 5%-10% better with lactate and glucose Exercise, inotropes and fast pacing: lactate uptake by myoc ...
Fritz Lipmann - Nobel Lecture
... rather specifically coupled with the glycolytic reaction. Here, however, we had found a coupling of phosphorylation with a respiratory system. This observation immediately suggested a rather sweeping biochemical significance, of transformations of electron transfer potential, respiratory or fermenta ...
... rather specifically coupled with the glycolytic reaction. Here, however, we had found a coupling of phosphorylation with a respiratory system. This observation immediately suggested a rather sweeping biochemical significance, of transformations of electron transfer potential, respiratory or fermenta ...
Rubisco
... inorganic phosphate for photophosphorylation ATP synthesis. It will also move NADPH synthesized by photorespiration into cytosol. NADPH will be converted to NADH during this process. ...
... inorganic phosphate for photophosphorylation ATP synthesis. It will also move NADPH synthesized by photorespiration into cytosol. NADPH will be converted to NADH during this process. ...
acyl-CoA
... uncontrolled diabetes) produce sufficient ketones in the blood to be effective as a fuel ketones are the preferred fuel if glucose, ketones, fatty acids all available in the blood primary tissues: using ketones, when available, are brain, muscle, kidney and intestine, but NOT the liver. -Hydroxybut ...
... uncontrolled diabetes) produce sufficient ketones in the blood to be effective as a fuel ketones are the preferred fuel if glucose, ketones, fatty acids all available in the blood primary tissues: using ketones, when available, are brain, muscle, kidney and intestine, but NOT the liver. -Hydroxybut ...
(i) C
... The NOESY experiment is crucial for the determination of protein structure. It uses the dipolar interaction of spins (the nuclear Overhauser effect, NOE) for correlation of protons. The intensity of the NOE is in first approximation proportional to 1/r6, with r being the distance between the protons ...
... The NOESY experiment is crucial for the determination of protein structure. It uses the dipolar interaction of spins (the nuclear Overhauser effect, NOE) for correlation of protons. The intensity of the NOE is in first approximation proportional to 1/r6, with r being the distance between the protons ...
Template-Synthesized Protein Nanotubes
... acid (APA), resulting in attachment (again via the phosphonate) of a monolayer of this molecule to the pore walls. The amino groups are then reacted with an excess quantity of the protein-immobilization agent glutaraldehyde (GA),14,15 leaving unreacted aldehyde groups on the pore walls. The membrane ...
... acid (APA), resulting in attachment (again via the phosphonate) of a monolayer of this molecule to the pore walls. The amino groups are then reacted with an excess quantity of the protein-immobilization agent glutaraldehyde (GA),14,15 leaving unreacted aldehyde groups on the pore walls. The membrane ...
The exam is worth 200 points, divided into 7 questions. You must do
... (d) (6 pts) Penicillin interferes with an essential bacterial growth process. What is this process (short answer, no structures)? How do resistant bacteria survive penicillin treatment? ...
... (d) (6 pts) Penicillin interferes with an essential bacterial growth process. What is this process (short answer, no structures)? How do resistant bacteria survive penicillin treatment? ...
Immobilized Enzyme Technology: Potentiality and Prospects
... 1g). It is different from other techniques in the sense that it does not require a support for the immobilization. There are two methods of cross linking in use, (i) Cross Linking Enzyme Aggregate (CLEA), and (ii) Cross Linking Enzyme Crystals (CLEC). Both CLEA and CLEC are modifications of a primit ...
... 1g). It is different from other techniques in the sense that it does not require a support for the immobilization. There are two methods of cross linking in use, (i) Cross Linking Enzyme Aggregate (CLEA), and (ii) Cross Linking Enzyme Crystals (CLEC). Both CLEA and CLEC are modifications of a primit ...
BI25M1
... becomes the C of pyruvate and oxaloacetate respectively [as we saw in Lecture 2]. For other amino-acids, the conversion involves several steps. ...
... becomes the C of pyruvate and oxaloacetate respectively [as we saw in Lecture 2]. For other amino-acids, the conversion involves several steps. ...
Fatty Acid Oxid - Univerzita Karlova v Praze
... Fatty acids esterified to CoA are substrates for the ER elongation machinery, which uses malonyl-CoA as donor of 2-carbon units. The reaction sequence is similar to Fatty Acid Synthase but individual steps are catalyzed by separate proteins. A family of enzymes designated Fatty Acid Elongases cata ...
... Fatty acids esterified to CoA are substrates for the ER elongation machinery, which uses malonyl-CoA as donor of 2-carbon units. The reaction sequence is similar to Fatty Acid Synthase but individual steps are catalyzed by separate proteins. A family of enzymes designated Fatty Acid Elongases cata ...
Fatty Acid Oxid
... Fatty acids esterified to CoA are substrates for the ER elongation machinery, which uses malonyl-CoA as donor of 2-carbon units. The reaction sequence is similar to Fatty Acid Synthase but individual steps are catalyzed by separate proteins. A family of enzymes designated Fatty Acid Elongases cata ...
... Fatty acids esterified to CoA are substrates for the ER elongation machinery, which uses malonyl-CoA as donor of 2-carbon units. The reaction sequence is similar to Fatty Acid Synthase but individual steps are catalyzed by separate proteins. A family of enzymes designated Fatty Acid Elongases cata ...
α-Ketoglutarate Dehydrogenase Activity Colorimetric Assay Kit
... α-Ketoglutarate Dehydrogenase (α-KGDH) (EC 1.2.4.2) is a key enzyme in the citric acid cycle. It forms an enzyme complex with dihydrolipoamide succinyl transferase (E2) and dihydrolipoamide dehydrogenase (E3). α-KGDH converts α-ketoglutarate into succinylCoA in the presence of NAD and CoA. It is hig ...
... α-Ketoglutarate Dehydrogenase (α-KGDH) (EC 1.2.4.2) is a key enzyme in the citric acid cycle. It forms an enzyme complex with dihydrolipoamide succinyl transferase (E2) and dihydrolipoamide dehydrogenase (E3). α-KGDH converts α-ketoglutarate into succinylCoA in the presence of NAD and CoA. It is hig ...
mammalian hibernation: biochemical adaptation
... The injurious effects of hypothermia on nonhibernating mammals arise from two main effects of cold on metabolic systems that have been optimized over millions of years of mammalian evolution for function within a narrow temperature window. The first is the differential effect of temperature change o ...
... The injurious effects of hypothermia on nonhibernating mammals arise from two main effects of cold on metabolic systems that have been optimized over millions of years of mammalian evolution for function within a narrow temperature window. The first is the differential effect of temperature change o ...
Biomarkery a mechanismy toxicity
... reacting with „nucleophiles“ in cells – i.e. electrone-rich sites (nucleotides, -NH2, -SH and others) 4) Only certain specific compounds selectively affect specific targets causing „specific“ toxicity (enzyme inhibitions – e.g. drugs, insecticides; receptor interactions – e.g. estrogens; effects at ...
... reacting with „nucleophiles“ in cells – i.e. electrone-rich sites (nucleotides, -NH2, -SH and others) 4) Only certain specific compounds selectively affect specific targets causing „specific“ toxicity (enzyme inhibitions – e.g. drugs, insecticides; receptor interactions – e.g. estrogens; effects at ...
Cytochromes P450 – importance of tissue specificity
... [14, 15] and transport through Golgi to the acrosome in male germ cells [15]. The exact reason for the static retention or recycling of cytochromes P450 is not fully understood. ER resident proteins must possess specific motifs, which enable them to be excluded from transport through Golgi to other ...
... [14, 15] and transport through Golgi to the acrosome in male germ cells [15]. The exact reason for the static retention or recycling of cytochromes P450 is not fully understood. ER resident proteins must possess specific motifs, which enable them to be excluded from transport through Golgi to other ...
Amino acid transport in Penicillium chrysogenum in relation to
... The second step in β-lactam synthesis is the oxidative cyclization of LLDACV into isopenicillin N (IPN). In this step, the bicyclic penam nucleus, consisting of the β-lactam and thiazolidine rings is generated (34, 102). This step is mediated by IPN synthase (IPNS) a protein of 38 kDa that is encode ...
... The second step in β-lactam synthesis is the oxidative cyclization of LLDACV into isopenicillin N (IPN). In this step, the bicyclic penam nucleus, consisting of the β-lactam and thiazolidine rings is generated (34, 102). This step is mediated by IPN synthase (IPNS) a protein of 38 kDa that is encode ...
Chem 11 Spring 2012 Practice Final
... 32) Which of the following is not true for a competitive inhibitor? A) It occupies the active site. B) It cannot be converted to products. C) It has a structure similar to the substrate. D) Increasing the substrate concentration can reverse competitive inhibition. E) It binds to the enzyme at a sit ...
... 32) Which of the following is not true for a competitive inhibitor? A) It occupies the active site. B) It cannot be converted to products. C) It has a structure similar to the substrate. D) Increasing the substrate concentration can reverse competitive inhibition. E) It binds to the enzyme at a sit ...
A generalized stoichiometric model of C3, C2, C2
... and oxygenation (VO, VC), and PEP carboxylation rate (VP). The ATP and NADPH requirements are SMA outputs and they are not related to light reactions at this stage. Reactions are typically grouped by the biochemical function of the pathways, of which only the entry point is calculated. The SMA is ba ...
... and oxygenation (VO, VC), and PEP carboxylation rate (VP). The ATP and NADPH requirements are SMA outputs and they are not related to light reactions at this stage. Reactions are typically grouped by the biochemical function of the pathways, of which only the entry point is calculated. The SMA is ba ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.