Chapter 9 Cellular Respiration (working)
... In exergonic reactions the energy stored in the reactants is greater than the energy stored in the products • Fermentation a catabolic process that makes a limited amount of ATP from glucose without an electron transport chain and that produces a characteristic end product, such as ethyl alcohol or ...
... In exergonic reactions the energy stored in the reactants is greater than the energy stored in the products • Fermentation a catabolic process that makes a limited amount of ATP from glucose without an electron transport chain and that produces a characteristic end product, such as ethyl alcohol or ...
Chapter 25 Chapter Topics Fatty Acid Biosynthesis
... works for heterozygotes because homozygotes have not receptor to stimulate. Elevated HDL seems to mitigate the plaque accumulation by acting to remove cholestrol from peripheral cells, returning it to the ...
... works for heterozygotes because homozygotes have not receptor to stimulate. Elevated HDL seems to mitigate the plaque accumulation by acting to remove cholestrol from peripheral cells, returning it to the ...
Chapter 7
... the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. ...
... the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. ...
Electrolytes and metabolic disorder.
... Normal energy supply for the heart: fatty acid oxidation, 10-40% energy derived from pyruvate (from glycolysis or conversion of lactate) FAs have higher yields of ATP/molecule, ATP yield/O2 molecule is 5%-10% better with lactate and glucose Exercise, inotropes and fast pacing: lactate uptake by myoc ...
... Normal energy supply for the heart: fatty acid oxidation, 10-40% energy derived from pyruvate (from glycolysis or conversion of lactate) FAs have higher yields of ATP/molecule, ATP yield/O2 molecule is 5%-10% better with lactate and glucose Exercise, inotropes and fast pacing: lactate uptake by myoc ...
17_Oxidative decarboxylation of pyruvate and Krebs cycle
... Pyruvate dehydrogenase complex is a classic example of multienzyme complex ...
... Pyruvate dehydrogenase complex is a classic example of multienzyme complex ...
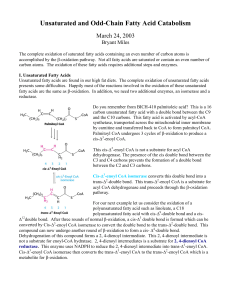
Unsaturated and Odd-Chain Fatty Acid Catabolism
... Most of the acetyl CoA produced by β-oxidation undergoes complete oxidation through the citric acid cycle. The entry of acetyl CoA into the citric acid cycle depends on the availability of oxaloacetate. Under fasting conditions or diabetes, gluconeogenisis depletes the concentration of oxaloacetate. ...
... Most of the acetyl CoA produced by β-oxidation undergoes complete oxidation through the citric acid cycle. The entry of acetyl CoA into the citric acid cycle depends on the availability of oxaloacetate. Under fasting conditions or diabetes, gluconeogenisis depletes the concentration of oxaloacetate. ...
Vitamin B12 deficiency, methylmalonic acidemia
... assayed for propionate and succinate oxidation, vitamin B12 (cobalamin) coenzyme biosynthesis, and methylmalonyl CoA mutase activity. Propionate oxidation was much lower than controls, but succinate oxidation was normal. Radioactivity from 57Co-labeled cobalamin was found in methyl cobalamin but not ...
... assayed for propionate and succinate oxidation, vitamin B12 (cobalamin) coenzyme biosynthesis, and methylmalonyl CoA mutase activity. Propionate oxidation was much lower than controls, but succinate oxidation was normal. Radioactivity from 57Co-labeled cobalamin was found in methyl cobalamin but not ...
Metabolism of Red Blood Cells (RBCs)
... • Hemoglobin, the chief protein of the red cells. • Other proteins are present in combination with lipids and oligosaccharide chains, forming the stroma and cell membrane. • Potassium, magnesium, and zinc concentrations in red cells are much higher than in the plasma. ...
... • Hemoglobin, the chief protein of the red cells. • Other proteins are present in combination with lipids and oligosaccharide chains, forming the stroma and cell membrane. • Potassium, magnesium, and zinc concentrations in red cells are much higher than in the plasma. ...
RBCs metabolism
... • Hemoglobin, the chief protein of the red cells. • Other proteins are present in combination with lipids and oligosaccharide chains, forming the stroma and cell membrane. • Potassium, magnesium, and zinc concentrations in red cells are much higher than in the plasma. ...
... • Hemoglobin, the chief protein of the red cells. • Other proteins are present in combination with lipids and oligosaccharide chains, forming the stroma and cell membrane. • Potassium, magnesium, and zinc concentrations in red cells are much higher than in the plasma. ...
NITROGEN METABOLISM: An Overview
... • NH3 from Brain is converted to Glutamine because of High activity of Glutamine Synthetase, • Glutamine so formed is transported in the blood to the Liver, ...
... • NH3 from Brain is converted to Glutamine because of High activity of Glutamine Synthetase, • Glutamine so formed is transported in the blood to the Liver, ...
Mechanistic model of cardiac energy metabolism predicts
... freely distributed, glycolysis can be considered localized in a subdomain within the cytosol. Unfortunately, at present, it is not feasible to measure dynamic changes in the fluxes and concentrations of key cytosolic and mitochondrial species in the transition from normal to ischemic conditions with ...
... freely distributed, glycolysis can be considered localized in a subdomain within the cytosol. Unfortunately, at present, it is not feasible to measure dynamic changes in the fluxes and concentrations of key cytosolic and mitochondrial species in the transition from normal to ischemic conditions with ...
Nutreval Interpretation Guide
... flow from the Carbohydrate Metabolism to the Energy Metabolism is inhibited, making it difficult to use carbohydrates as fuel in the mitochondria. This could be due to deficiencies of lipoic acid, B1, ...
... flow from the Carbohydrate Metabolism to the Energy Metabolism is inhibited, making it difficult to use carbohydrates as fuel in the mitochondria. This could be due to deficiencies of lipoic acid, B1, ...
Increase of Melanogenesis in the Presence of Fatty Acids
... active site. This conformational change enhances the affinity of active site towards its substrate which results in a more kinetically favorable reaction. On the other hand, conformational studies have revealed that, in addition to its active site, tyrosinase posses an effector site to which the eff ...
... active site. This conformational change enhances the affinity of active site towards its substrate which results in a more kinetically favorable reaction. On the other hand, conformational studies have revealed that, in addition to its active site, tyrosinase posses an effector site to which the eff ...
Adv. Bio. Ch 9 Glyco and Resp
... e- from NADH and FADH2 “fall down” the ETC with oxygen being the final e- acceptor and H2O being ...
... e- from NADH and FADH2 “fall down” the ETC with oxygen being the final e- acceptor and H2O being ...
here - Solve ME/CFS Initiative
... Similarities to ME/CFS. Infection or stressor (both) Cytokine release (sepsis) Oxidative stress (both, more in sepsis) Mitochondrial dysfunction at oxidative phosphorylation site (both) Low blood volume (both) Hypometabolism (starvation) Hyperglycemia (starvation) Mitochondria use lipids and amino a ...
... Similarities to ME/CFS. Infection or stressor (both) Cytokine release (sepsis) Oxidative stress (both, more in sepsis) Mitochondrial dysfunction at oxidative phosphorylation site (both) Low blood volume (both) Hypometabolism (starvation) Hyperglycemia (starvation) Mitochondria use lipids and amino a ...
amino acids properties
... 2-They have a high melting point reflecting the high energy needed to break the ionic forces maintaining the crystal lattice. It is important to note that the general properties of amino acids is shared by all the amino acids and is in many cases contributed by its α-amino and αcarboxyl group . Amin ...
... 2-They have a high melting point reflecting the high energy needed to break the ionic forces maintaining the crystal lattice. It is important to note that the general properties of amino acids is shared by all the amino acids and is in many cases contributed by its α-amino and αcarboxyl group . Amin ...
Urea Cycle - MBBS Students Club
... • The ammonia produced by enteric bacteria and absorbedinto portal venous blood and the ammonia produced by tissues are rapidly removed from circulation by the liver and converted to urea. • Only traces (10–20μg/dL) thus normally are present in peripheral blood. • This is essential, since ammonia is ...
... • The ammonia produced by enteric bacteria and absorbedinto portal venous blood and the ammonia produced by tissues are rapidly removed from circulation by the liver and converted to urea. • Only traces (10–20μg/dL) thus normally are present in peripheral blood. • This is essential, since ammonia is ...
File - Mrs. LeCompte
... o This creates potential energy o ATP Synthase uses the potential energy stored in the proton gradient to make ATP by allowing H+s to diffuse through it back across the membrane o The drop in potential energy can then be captured in the bonds that attach the last phosphate to ADP ATP. ...
... o This creates potential energy o ATP Synthase uses the potential energy stored in the proton gradient to make ATP by allowing H+s to diffuse through it back across the membrane o The drop in potential energy can then be captured in the bonds that attach the last phosphate to ADP ATP. ...
Slide 1
... Energy released by the oxidation (controlled burning) of carbohydrates and fats, and energy harvested by photosynthesis in green plants, are channeled into making the molecule adenosine triphosphate (ATP). ATP is a high energy compound considered to be the universal currency of biological energy. On ...
... Energy released by the oxidation (controlled burning) of carbohydrates and fats, and energy harvested by photosynthesis in green plants, are channeled into making the molecule adenosine triphosphate (ATP). ATP is a high energy compound considered to be the universal currency of biological energy. On ...
Hydrogen peroxide production regulates the mitochondrial
... skeletal muscle. The mechanism behind this biochemical process was known as the glucose–fatty acid cycle. Under such condition, the elevated content of acetyl-CoA inhibits pyruvate dehydrogenase complex (PDH) activity via activation of PDH kinase, the enzyme responsible for phosphorylation and inact ...
... skeletal muscle. The mechanism behind this biochemical process was known as the glucose–fatty acid cycle. Under such condition, the elevated content of acetyl-CoA inhibits pyruvate dehydrogenase complex (PDH) activity via activation of PDH kinase, the enzyme responsible for phosphorylation and inact ...
Lecture 33 - University of Arizona
... cAMP triggers two types of phosphorylation circuits in muscle cells; one that stimulates glycogen degradation and a second that inhibits glycogen synthesis. ...
... cAMP triggers two types of phosphorylation circuits in muscle cells; one that stimulates glycogen degradation and a second that inhibits glycogen synthesis. ...
Ketosis

Ketosis /kɨˈtoʊsɨs/ is a metabolic state where most of the body's energy supply comes from ketone bodies in the blood, in contrast to a state of glycolysis where blood glucose provides most of the energy. It is characterised by serum concentrations of ketone bodies over 0.5 millimolar, with low and stable levels of insulin and blood glucose. It is almost always generalized with hyperketonemia, that is, an elevated level of ketone bodies in the blood throughout the body. Ketone bodies are formed by ketogenesis when liver glycogen stores are depleted (or from metabolising medium-chain triglycerides). The main ketone bodies used for energy are acetoacetate and β-hydroxybutyrate, and the levels of ketone bodies are regulated mainly by insulin and glucagon. Most cells in the body can use both glucose and ketone bodies for fuel, and during ketosis, free fatty acids and glucose synthesis (gluconeogenesis) fuel the remainder.Longer-term ketosis may result from fasting or staying on a low-carbohydrate diet, and deliberately induced ketosis serves as a medical intervention for intractable epilepsy. In glycolysis, higher levels of insulin promote storage of body fat and block release of fat from adipose tissues, while in ketosis, fat reserves are readily released and consumed. For this reason, ketosis is sometimes referred to as the body's ""fat burning"" mode.