Atomic Orbitals and quantum numbers
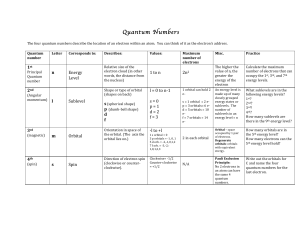
... Magnetic Quantum Number, ml •Describes the three-dimensional orientation of the orbital. •Values are integers ranging from -l to l: −l ≤ ml ≤ l. •Therefore, on any given energy level, there can be up to 1s orbital, 3p orbitals, 5d orbitals, and 7f orbitals. ...
... Magnetic Quantum Number, ml •Describes the three-dimensional orientation of the orbital. •Values are integers ranging from -l to l: −l ≤ ml ≤ l. •Therefore, on any given energy level, there can be up to 1s orbital, 3p orbitals, 5d orbitals, and 7f orbitals. ...
science 1 small-group tutorial scheme
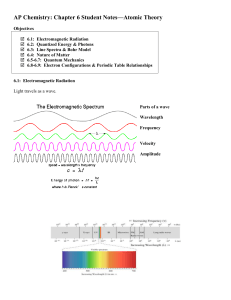
... The hydrogen emission spectrum experiment gives rise to a line spectrum. What does this tell us about the electromagnetic radiation being given out by the hydrogen atom? How was this result explained in terms of the simple Quantum Mechanical (Bohr) model of atomic structure? ...
... The hydrogen emission spectrum experiment gives rise to a line spectrum. What does this tell us about the electromagnetic radiation being given out by the hydrogen atom? How was this result explained in terms of the simple Quantum Mechanical (Bohr) model of atomic structure? ...
Chapter 4 Arrangement of Electrons in Atoms
... • Explain how the Heisenberg uncertainty principle and the Schrödinger wave equation led to the idea of atomic orbitals. • List the four quantum numbers and describe their significance. • Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per ...
... • Explain how the Heisenberg uncertainty principle and the Schrödinger wave equation led to the idea of atomic orbitals. • List the four quantum numbers and describe their significance. • Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per ...
Electron Configuration Notes
... The line can slant either way; one way, it's spinning one direction, the other slant represents an electron spinning the other way; W or Ø . Two electrons in an orbital need both diagonal lines. U Let's take an example; Na, sodium, element #11. Electron Configuration: 1s 2 2s 2 2p 6 3s 1 Its orbital ...
... The line can slant either way; one way, it's spinning one direction, the other slant represents an electron spinning the other way; W or Ø . Two electrons in an orbital need both diagonal lines. U Let's take an example; Na, sodium, element #11. Electron Configuration: 1s 2 2s 2 2p 6 3s 1 Its orbital ...
WAVE MECHANICS AND QUANTUM NUMBERS
... equation describing the location and energy of an electron in a hydrogen atom. 2. Modern day quantum mechanical model comes from the math solutions to Schrödinger’s equations. It extended de Broglie’s work by considering the movement of a particle in an electromagnetic field. 3. Defined quantum numb ...
... equation describing the location and energy of an electron in a hydrogen atom. 2. Modern day quantum mechanical model comes from the math solutions to Schrödinger’s equations. It extended de Broglie’s work by considering the movement of a particle in an electromagnetic field. 3. Defined quantum numb ...
Chapt7
... related to spatial orientation of orbitals within a given subshell possible values of ml = - l, ..... 0, ....., + l the number of ml values = number of orbitals within a subshell e.g., within a subshell having l = 2, there are 5 orbitals corresponding to the 5 possible values of ml ( - 2, -1, 0, +1, ...
... related to spatial orientation of orbitals within a given subshell possible values of ml = - l, ..... 0, ....., + l the number of ml values = number of orbitals within a subshell e.g., within a subshell having l = 2, there are 5 orbitals corresponding to the 5 possible values of ml ( - 2, -1, 0, +1, ...
Quiz 1 Key
... hydrogen had only certain colors and thus certain wavelengths present. Because wavelength is related to energy, this indicated that there were only certain energies of light emitted. This indicated that there were only defined energy levels for an excited atom and the electrons could only be at cert ...
... hydrogen had only certain colors and thus certain wavelengths present. Because wavelength is related to energy, this indicated that there were only certain energies of light emitted. This indicated that there were only defined energy levels for an excited atom and the electrons could only be at cert ...
Which scientist developed the quantum mechanical model of the
... A.) Bohr B.) Rutherford C.) Schrodinger D.) Heisenberg ...
... A.) Bohr B.) Rutherford C.) Schrodinger D.) Heisenberg ...
Review Notes - Biochemistry
... 5. Chemical Formula: Where each _ELEMENT_ is represented by its chemical _SYMBOL_ and the _NUMBER__ of atoms is shown in __SUBSCRIPTS__. ...
... 5. Chemical Formula: Where each _ELEMENT_ is represented by its chemical _SYMBOL_ and the _NUMBER__ of atoms is shown in __SUBSCRIPTS__. ...
Unit 2: Atoms and their Electrons
... d orbitals, l = 2 Aufbau means from bottom up – orbitals are filled from the lowest energy level first and then up, Pauli Exclusion principle states that no two electrons in an atom can have all four quantum numbers the same, Hund’s rule states that electrons do not pair up to fill an orbital in a s ...
... d orbitals, l = 2 Aufbau means from bottom up – orbitals are filled from the lowest energy level first and then up, Pauli Exclusion principle states that no two electrons in an atom can have all four quantum numbers the same, Hund’s rule states that electrons do not pair up to fill an orbital in a s ...
Answers to Critical Thinking Questions 4
... a) 1s22s22p63s23p44s1 – the 3p orbitals were not completely filled before electrons were added to 4s (violating the Aufbau principle). The correct configuration is 1s22s22p63s23p5 b) 1s22s22p63s23p7 – the maximum number of electrons in 3p is 6 (violating the Pauli exclusion principle). The correct c ...
... a) 1s22s22p63s23p44s1 – the 3p orbitals were not completely filled before electrons were added to 4s (violating the Aufbau principle). The correct configuration is 1s22s22p63s23p5 b) 1s22s22p63s23p7 – the maximum number of electrons in 3p is 6 (violating the Pauli exclusion principle). The correct c ...
Electron Configurations
... So you learned about the Bohr model of an atom as well the electronic configuration of that atom. If you have taken or are taking any sort of an advanced chemistry class, then you probably didn’t have much trouble with these concepts. Otherwise, you may want some extra information on the subject. Mo ...
... So you learned about the Bohr model of an atom as well the electronic configuration of that atom. If you have taken or are taking any sort of an advanced chemistry class, then you probably didn’t have much trouble with these concepts. Otherwise, you may want some extra information on the subject. Mo ...
CM1111* Question 1 (40 marks) Multiple Choice Questions, 5 marks
... (4) Which of the following statement(s) is/are true? A. In order of increasing ionisation energy, Mg
... (4) Which of the following statement(s) is/are true? A. In order of increasing ionisation energy, Mg
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.