L-J Chemistry 1 Quiz 25 1 A property that depends on the amount of
... A property that depends on the amount of matter Research carried out to solve a specific problem A cube that is 10 dm on each side =(equals 1 liter) Atomic mass of a substance in grams Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lo ...
... A property that depends on the amount of matter Research carried out to solve a specific problem A cube that is 10 dm on each side =(equals 1 liter) Atomic mass of a substance in grams Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lo ...
Tuesday Aug 19
... Objective: Write the electronic configuration of any element. Checkpoint: • How many different photons of light can an atom give off if it has 4 energy levels? HW: Emission spectrum lab (due Tuesday) ...
... Objective: Write the electronic configuration of any element. Checkpoint: • How many different photons of light can an atom give off if it has 4 energy levels? HW: Emission spectrum lab (due Tuesday) ...
Arrangement of Electrons In Atoms
... Indicate the shape of the orbital Also called sublevels Number of shapes equal to n Value of l are zero and all positive integers less than or equal to (n - 1) Example: n = 2; l = 0 or l = 1 • Each integer is assigned a letter Example: 0 = s; 1 = p; 2 = d; 3 = f • n = 2; there are two sublevels s an ...
... Indicate the shape of the orbital Also called sublevels Number of shapes equal to n Value of l are zero and all positive integers less than or equal to (n - 1) Example: n = 2; l = 0 or l = 1 • Each integer is assigned a letter Example: 0 = s; 1 = p; 2 = d; 3 = f • n = 2; there are two sublevels s an ...
Quantum Mechanical Model
... Quantum Mechanical Model • As the energy of an electron increases, so does the quantum number (n) • Each principle energy level is also split up into one or more sublevels • Chart on Pg. 145 [http://www.chemistry.mcmaster.ca/esam/Chapter_4/fig4-2.jpg] ...
... Quantum Mechanical Model • As the energy of an electron increases, so does the quantum number (n) • Each principle energy level is also split up into one or more sublevels • Chart on Pg. 145 [http://www.chemistry.mcmaster.ca/esam/Chapter_4/fig4-2.jpg] ...
4-1 The lowest energy state of an atom is its ground state. (usually
... Quantum numbers specify the properties of atomic orbital and the properties of electrons in orbitals. Ex: fluorine 1s2 2s2 2px2py2pz1 The principal quantum number (n) indicates the main energy level occupied by the electron Ex: 1s2 2s2 2px2py2pz1 The angular momentum quantum number indicates the sha ...
... Quantum numbers specify the properties of atomic orbital and the properties of electrons in orbitals. Ex: fluorine 1s2 2s2 2px2py2pz1 The principal quantum number (n) indicates the main energy level occupied by the electron Ex: 1s2 2s2 2px2py2pz1 The angular momentum quantum number indicates the sha ...
summary sheet
... SUMMARY Electrons in Atoms (Section 29.1) The Bohr model fails to fully describe atoms because it combines elements of classical physics with some principles of quantum mechanics. To explain the observed properties of an atom, electrons must be described with a wave function that is determined by so ...
... SUMMARY Electrons in Atoms (Section 29.1) The Bohr model fails to fully describe atoms because it combines elements of classical physics with some principles of quantum mechanics. To explain the observed properties of an atom, electrons must be described with a wave function that is determined by so ...
Electron Configurations
... – All orbitals within a sublevel have equal energy. (All 3 p sublevels are equal energy at any level.) – Energy sublevels within a principle energy level have different energies (s
... – All orbitals within a sublevel have equal energy. (All 3 p sublevels are equal energy at any level.) – Energy sublevels within a principle energy level have different energies (s
Midterm Exam 2
... Oxygen and nitrogen are two of the major components of the atmosphere as well as critical components for all biological system. Both elements exist as diatomic gases (X2) within the atmosphere. O2 is a reactive species which is often involved in oxidation. This process leads to the formation or rust ...
... Oxygen and nitrogen are two of the major components of the atmosphere as well as critical components for all biological system. Both elements exist as diatomic gases (X2) within the atmosphere. O2 is a reactive species which is often involved in oxidation. This process leads to the formation or rust ...
Modern Model of the Atom
... aufbau principle: electrons fill the lowest energy orbitals first Pauli exclusion principle: no two electrons in an atom can have the same four quantum numbers Hund’s rule: every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons ...
... aufbau principle: electrons fill the lowest energy orbitals first Pauli exclusion principle: no two electrons in an atom can have the same four quantum numbers Hund’s rule: every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons ...
Atomic Structure
... 4 lobes oriented about x,y,z axes (dxy, dyz, dxz, dx2y2, dz2) f orbital = 3 dumbells – 7 possible 6 lobes oriented about x,y,z axes (fxyz, etc) ...
... 4 lobes oriented about x,y,z axes (dxy, dyz, dxz, dx2y2, dz2) f orbital = 3 dumbells – 7 possible 6 lobes oriented about x,y,z axes (fxyz, etc) ...
MIDTERM REVIEW GAME 16-17
... Were used to bombard thin metal foil. Were used to bombard a cathode plate. Collided with electrons. ...
... Were used to bombard thin metal foil. Were used to bombard a cathode plate. Collided with electrons. ...
Atomic Structure Practice Answers
... 5. This is a possible configuration for a transition metal atom. B 6. This electron configuration is not possible. A 7. This is a possible configuration for a transition metal ion. C 8-11 refer to the following: A. B. C. D. E. ...
... 5. This is a possible configuration for a transition metal atom. B 6. This electron configuration is not possible. A 7. This is a possible configuration for a transition metal ion. C 8-11 refer to the following: A. B. C. D. E. ...
Quantum Atom
... Wave function (Ψ2) – series of solutions that describes the allowed energy levels for electrons Shows regions of probability of finding an electron Regions of high electron density have large values of Ψ2 ...
... Wave function (Ψ2) – series of solutions that describes the allowed energy levels for electrons Shows regions of probability of finding an electron Regions of high electron density have large values of Ψ2 ...
VSEPR Molecular Geometry VSEPR Molecular Geometry
... Add total # of valence electrons Determine central atom, eletropositive element Determine terminal/ peripheral atoms Connect central and terminal atoms Fulfill octet rule for terminal atoms Add electron to the central atom Determine the possibility of multiple bonds ...
... Add total # of valence electrons Determine central atom, eletropositive element Determine terminal/ peripheral atoms Connect central and terminal atoms Fulfill octet rule for terminal atoms Add electron to the central atom Determine the possibility of multiple bonds ...
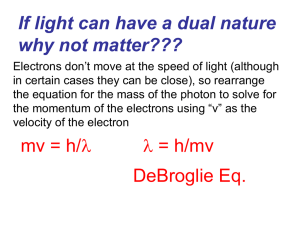
Slide 1
... If light can have a dual nature why not matter??? Electrons don’t move at the speed of light (although in certain cases they can be close), so rearrange the equation for the mass of the photon to solve for the momentum of the electrons using “v” as the velocity of the electron ...
... If light can have a dual nature why not matter??? Electrons don’t move at the speed of light (although in certain cases they can be close), so rearrange the equation for the mass of the photon to solve for the momentum of the electrons using “v” as the velocity of the electron ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.