Primary electrons make random elastic and inelastic collision either
... Bremsstrahlung (continuum) X-rays, deceleration of primary electrons (with primary energy E0) in the Columbic field of the nucleus, a type of ‘braking’ effect…. i.e. as to pass though the stronger electric filed, close to nuclei, it will suffer a “quantum jump” to a low energy state, which will make ...
... Bremsstrahlung (continuum) X-rays, deceleration of primary electrons (with primary energy E0) in the Columbic field of the nucleus, a type of ‘braking’ effect…. i.e. as to pass though the stronger electric filed, close to nuclei, it will suffer a “quantum jump” to a low energy state, which will make ...
CHEMISTRY MIDTERM REVIEW
... 48. Draw the Lewis dot structures for the following: CH4, PF3, C2H2, O3, I3-,NO349. Write the shape of each molecule in the previous question next to its Lewis dot structure. 50. Why do atoms share electrons in covalent bonds? 51. How many lone pairs of electrons are there in the NH3 molecule? ...
... 48. Draw the Lewis dot structures for the following: CH4, PF3, C2H2, O3, I3-,NO349. Write the shape of each molecule in the previous question next to its Lewis dot structure. 50. Why do atoms share electrons in covalent bonds? 51. How many lone pairs of electrons are there in the NH3 molecule? ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... 28. The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A. Pauli Exclusion Principle B. Hund’s Rule C. deBroglie hypothesis D. Heisenberg Uncertainty Principle 29. Which electron configuration correctly denotes an at ...
... 28. The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A. Pauli Exclusion Principle B. Hund’s Rule C. deBroglie hypothesis D. Heisenberg Uncertainty Principle 29. Which electron configuration correctly denotes an at ...
Honors Chemistry First Marking Period Review Sheet
... four quantum numbers. I understand that electrons are assigned to the subshells of an atom in order of increasing subshell energy. In the H atom, the subshell energies increase with increasing n , but in a many-electron atom, the energies depend on both n and l. I can use the Aufbau principle to hel ...
... four quantum numbers. I understand that electrons are assigned to the subshells of an atom in order of increasing subshell energy. In the H atom, the subshell energies increase with increasing n , but in a many-electron atom, the energies depend on both n and l. I can use the Aufbau principle to hel ...
Chapter 3
... b. Explain Rutherford’s gold foil experiment and it’s significance c. Explain atomic spectra and it’s significance to Bohr’s model 2. Quantum Mechanics: a. The 4 quantum numbers and what they describe b. The difference between orbits (Bohr) and orbitals c. Pauli’s exclusion principle (no two electro ...
... b. Explain Rutherford’s gold foil experiment and it’s significance c. Explain atomic spectra and it’s significance to Bohr’s model 2. Quantum Mechanics: a. The 4 quantum numbers and what they describe b. The difference between orbits (Bohr) and orbitals c. Pauli’s exclusion principle (no two electro ...
Atomic Structure MC Review_ corrected
... 28. The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A. Pauli Exclusion Principle B. Hund’s Rule C. deBroglie hypothesis D. Heisenberg Uncertainty Principle 29. Which electron configuration correctly denotes an at ...
... 28. The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A. Pauli Exclusion Principle B. Hund’s Rule C. deBroglie hypothesis D. Heisenberg Uncertainty Principle 29. Which electron configuration correctly denotes an at ...
Quantum Numbers and Electronic Configuration
... The Aufbau (building up) Principle for Electronic Configuration. In the so-called “ground state” of an atom the electronic configuration generally follows this principle. According to this principle, electrons enter into states in order of the states increasing energy. Electrons are reluctant to pai ...
... The Aufbau (building up) Principle for Electronic Configuration. In the so-called “ground state” of an atom the electronic configuration generally follows this principle. According to this principle, electrons enter into states in order of the states increasing energy. Electrons are reluctant to pai ...
AP Chemistry Chapter 7 Review Packet
... The orange-yellow color of sodium-vapor streetlights results from electrons in sodium atoms falling from 3p to 3s orbitals. The wavelength of one orange-yellow line in the spectrum of sodium is 589 nm. a. Write the electron configuration for the ground state of sodium. b. Write the electron configur ...
... The orange-yellow color of sodium-vapor streetlights results from electrons in sodium atoms falling from 3p to 3s orbitals. The wavelength of one orange-yellow line in the spectrum of sodium is 589 nm. a. Write the electron configuration for the ground state of sodium. b. Write the electron configur ...
3.4oquantum.4u
... we cannot specify exact orbits. Another problem is when an electron changes energy levels during the emission of atomic spectra. ...
... we cannot specify exact orbits. Another problem is when an electron changes energy levels during the emission of atomic spectra. ...
chemistry-study-guide-grade
... 1. Provide the meaning of each type of quantum number (principal, angular momentum, magnetic and electron spin). 2. Apply quantum number rules to determine allowable values for each type of quantum number. 3. Understand the basis of atomic orbitals. 4. Arrange atomic orbitals based upon energy level ...
... 1. Provide the meaning of each type of quantum number (principal, angular momentum, magnetic and electron spin). 2. Apply quantum number rules to determine allowable values for each type of quantum number. 3. Understand the basis of atomic orbitals. 4. Arrange atomic orbitals based upon energy level ...
Slide 1 - s3.amazonaws.com
... Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation. In 1924, Louis de Bro ...
... Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation. In 1924, Louis de Bro ...
1 - theozone
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
1 - Revsworld
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
Electron Configurations
... • We can only describe the probable locations of electrons. • We will describe the location of electrons when the atom is at its lowest energy . • These are called “ground state” configurations. • If electrons move to higher energy states this is called “excited.” ...
... • We can only describe the probable locations of electrons. • We will describe the location of electrons when the atom is at its lowest energy . • These are called “ground state” configurations. • If electrons move to higher energy states this is called “excited.” ...
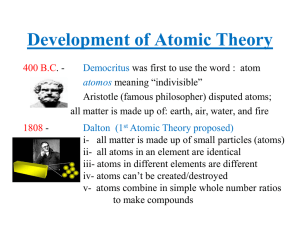
Atomic Structure 1. Historical perspective of the model of the atom a
... a.) In 1803, John Dalton proposed the atomic theory which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or ...
... a.) In 1803, John Dalton proposed the atomic theory which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or ...
Powerpoint handout
... Bohr derived a more general formula to predict the observed energies of light: Each electron’s energy is determined by which level it is in. The levels are designated by whole numbers, n. ...
... Bohr derived a more general formula to predict the observed energies of light: Each electron’s energy is determined by which level it is in. The levels are designated by whole numbers, n. ...
Exam 2-1
... the electron’s angular momentum quantum number. the presence of other electrons between the electron and the nucleus reducing the attraction. the number of unpaired electrons that have the same spin. none of the above. ...
... the electron’s angular momentum quantum number. the presence of other electrons between the electron and the nucleus reducing the attraction. the number of unpaired electrons that have the same spin. none of the above. ...
CHEMISTRY 1A
... at STP. Determine the empirical formula. (!! For 5 extra points determine the molecular formula of the ...
... at STP. Determine the empirical formula. (!! For 5 extra points determine the molecular formula of the ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.