Chemistry: The Nature of Matter
... More distant an electron is from the nucleus, the greater the energy possible in the atom Different states of energy are called energy levels or electron shells o 1st shell is closest to the nucleus, has the lowest energy, and holds only 2 electrons o 2nd shell has a little more energy and holds ...
... More distant an electron is from the nucleus, the greater the energy possible in the atom Different states of energy are called energy levels or electron shells o 1st shell is closest to the nucleus, has the lowest energy, and holds only 2 electrons o 2nd shell has a little more energy and holds ...
Exam 1 Topics to Review (McMurry Chpts 1
... c. Understand definitions of shells, subshells, orbitals, and electrons, and how they are organized. d. Understand that bigger the shell number à higher the energy and bigger the size of shell. e. Be familiar with general shapes of s, p, and d orbitals. f. Know the number of orbitals in each ty ...
... c. Understand definitions of shells, subshells, orbitals, and electrons, and how they are organized. d. Understand that bigger the shell number à higher the energy and bigger the size of shell. e. Be familiar with general shapes of s, p, and d orbitals. f. Know the number of orbitals in each ty ...
Chem 1st Sem Rev Ch2
... c. father of the modern atomic theory, everything made of atoms d. planetary model of the atom, electrons move around the nucleus like planets around sun. e. plum pudding model of the atom: atom looks like chocolate chip cookie f. gold foil experiment – atoms have a dense core called nucleus g. he g ...
... c. father of the modern atomic theory, everything made of atoms d. planetary model of the atom, electrons move around the nucleus like planets around sun. e. plum pudding model of the atom: atom looks like chocolate chip cookie f. gold foil experiment – atoms have a dense core called nucleus g. he g ...
WAVE MECHANICS (Schrödinger, 1926)
... mechanics which takes into account the wave nature of matter and the uncertainty principle. * The state of an electron is described by a function y, called the “wave function”. * y can be obtained by solving Schrödinger’s equation (a differential equation): Hy=Ey ...
... mechanics which takes into account the wave nature of matter and the uncertainty principle. * The state of an electron is described by a function y, called the “wave function”. * y can be obtained by solving Schrödinger’s equation (a differential equation): Hy=Ey ...
Chemistry CPA Activity Sheet Week of November 18, 2013 Unit
... Discuss the dual wave-particle nature of light. Discuss the significance of the photoelectric effect and the line-emission spectrum of hydrogen to the development of the atomic model. Discuss Louis de Broglie’s role in the development of the quantum model of the atom. Explain how the Heisenberg unce ...
... Discuss the dual wave-particle nature of light. Discuss the significance of the photoelectric effect and the line-emission spectrum of hydrogen to the development of the atomic model. Discuss Louis de Broglie’s role in the development of the quantum model of the atom. Explain how the Heisenberg unce ...
GROUP QUIZ UNIT 04 NAMES I. Fill in the charts (1 point per blank
... ____13. The Lewis-dot notation for an element in the third period is represented by a symbol surrounded by three dots. The electron configuration for this element is a. 1s22s22p63s23p1. b. 1s22s22p63s13p2. c. 1s22s22p53s23p2. d. 1s22s22p63s2. ____14. The Lewis-dot notation for fluorine would show a. ...
... ____13. The Lewis-dot notation for an element in the third period is represented by a symbol surrounded by three dots. The electron configuration for this element is a. 1s22s22p63s23p1. b. 1s22s22p63s13p2. c. 1s22s22p53s23p2. d. 1s22s22p63s2. ____14. The Lewis-dot notation for fluorine would show a. ...
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 1. The Heisenberg uncertainty principle states that a. electrons have no momentum b. the position of an electron is impossible to determine c. the faster an electron moves, the more unreliable is its energy d. the momentum and the position of an electron cannot be precisely defined simultaneous ...
... ____ 1. The Heisenberg uncertainty principle states that a. electrons have no momentum b. the position of an electron is impossible to determine c. the faster an electron moves, the more unreliable is its energy d. the momentum and the position of an electron cannot be precisely defined simultaneous ...
Chemistry Questions
... 3. The positively charged particle in the nucleus of an atom is 4. What is the total number of electrons in the nucleus of an atom of potassium-42? 5. Which of the following elements have the greatest number of neutrons? a. 37Cl b. 39K 4. An atomic mass unit is defined as exactly a. 1/16 the mass of ...
... 3. The positively charged particle in the nucleus of an atom is 4. What is the total number of electrons in the nucleus of an atom of potassium-42? 5. Which of the following elements have the greatest number of neutrons? a. 37Cl b. 39K 4. An atomic mass unit is defined as exactly a. 1/16 the mass of ...
Midterm Review Sample Content Questions
... 35. Identify which of the following sets of quantum numbers cannot occur for an electron. Identify the error and why it is incorrect. If it is correct identify the proper configuration value, i.e. 3d2. a. n=3 l= 3 m l = -2 ms = +1/2 c. n=3 l= 2 m l = -1 ms = -1/2 b. n=2 l= 1 m l = -0 ms = -1/2 ...
... 35. Identify which of the following sets of quantum numbers cannot occur for an electron. Identify the error and why it is incorrect. If it is correct identify the proper configuration value, i.e. 3d2. a. n=3 l= 3 m l = -2 ms = +1/2 c. n=3 l= 2 m l = -1 ms = -1/2 b. n=2 l= 1 m l = -0 ms = -1/2 ...
Quantum number
... Orbital Filling Chart This diagrams shows how the energy of the orbitals can overlap. ...
... Orbital Filling Chart This diagrams shows how the energy of the orbitals can overlap. ...
Name
... orbital until all the orbitals contain one electron with the same spin direction. b. Electrons occupy orbitals of lowest energy first. c. An atomic orbital may describe at most two electrons. ...
... orbital until all the orbitals contain one electron with the same spin direction. b. Electrons occupy orbitals of lowest energy first. c. An atomic orbital may describe at most two electrons. ...
Quantum Numbers Practice Problems Name: AP Physics Period: 1
... 2. According to the deBroglie theory, electrons orbiting around the nucleus of an atom are waves (as well as particles). How does our understanding of electron waves change when we give up the idea of a simple orbit for an electron (like the ones that Bohr envisioned)? ...
... 2. According to the deBroglie theory, electrons orbiting around the nucleus of an atom are waves (as well as particles). How does our understanding of electron waves change when we give up the idea of a simple orbit for an electron (like the ones that Bohr envisioned)? ...
Test 1 Guide
... 1) Neutrons have almost no mass (in amu) and no charge. 2) The fact that 2 different elements may have atoms with the same atomic mass violates Dalton’s atomic theory. 3) Deuterium differs from hydrogen in the number of protons it has. 4) In the quantum mechanical model of the atom, electrons are co ...
... 1) Neutrons have almost no mass (in amu) and no charge. 2) The fact that 2 different elements may have atoms with the same atomic mass violates Dalton’s atomic theory. 3) Deuterium differs from hydrogen in the number of protons it has. 4) In the quantum mechanical model of the atom, electrons are co ...
Section 3.7
... 6. Statistics are used to predict situations such as the number of megajoules of electricity that will be used in a city in a given winter month, or the number of students that will achieve honours on a national examination, or the number of cases of influenza that will occur over the winter in a co ...
... 6. Statistics are used to predict situations such as the number of megajoules of electricity that will be used in a city in a given winter month, or the number of students that will achieve honours on a national examination, or the number of cases of influenza that will occur over the winter in a co ...
Slide 1
... Orbital Filling Chart This diagrams shows how the energy of the orbitals can overlap. ...
... Orbital Filling Chart This diagrams shows how the energy of the orbitals can overlap. ...
File
... electrons – each of the 3 orbitals can hold 2 electrons • d orbitals can hold 10 electrons – each of the 5 orbitals can hold 2 electrons • f orbitals can hold 14 electrons – each of the 7 orbitals can hold 2 electrons ...
... electrons – each of the 3 orbitals can hold 2 electrons • d orbitals can hold 10 electrons – each of the 5 orbitals can hold 2 electrons • f orbitals can hold 14 electrons – each of the 7 orbitals can hold 2 electrons ...
Midterm review
... o General orbital energy order: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 5p Exceptions: Neutral atoms: Cr, Cu, Nb, Mo, Ions: all transition metals when they ionize the first two electrons that are lost are from the ns shell not the (n-1)d shell. Filling orbitals 1. Pauli Principle: Every elect ...
... o General orbital energy order: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 5p Exceptions: Neutral atoms: Cr, Cu, Nb, Mo, Ions: all transition metals when they ionize the first two electrons that are lost are from the ns shell not the (n-1)d shell. Filling orbitals 1. Pauli Principle: Every elect ...
How are quantum numbers used to describe electrons
... How many orbitals in the 4th energy level? How many electrons can be in the 4th energy level? For the known elements, ________ orbitals and ______ electrons is the maximum number in energy levels 5-7. What rules are used to explain how electrons fill orbitals? Pauli exclusion principle—no two electr ...
... How many orbitals in the 4th energy level? How many electrons can be in the 4th energy level? For the known elements, ________ orbitals and ______ electrons is the maximum number in energy levels 5-7. What rules are used to explain how electrons fill orbitals? Pauli exclusion principle—no two electr ...
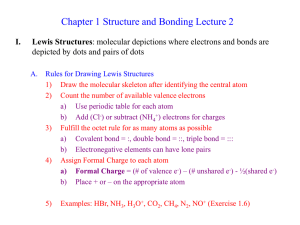
Ch 1 Lecture 2
... 2) Count the number of available valence electrons a) Use periodic table for each atom b) Add (Cl-) or subtract (NH4+) electrons for charges 3) Fulfill the octet rule for as many atoms as possible a) Covalent bond = :, double bond = ::, triple bond = ::: b) Electronegative elements can have lone pai ...
... 2) Count the number of available valence electrons a) Use periodic table for each atom b) Add (Cl-) or subtract (NH4+) electrons for charges 3) Fulfill the octet rule for as many atoms as possible a) Covalent bond = :, double bond = ::, triple bond = ::: b) Electronegative elements can have lone pai ...
WORKSHEET 36: ATOMIC PROPERTIES
... Explain your answer. (2) _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ 7. Define electron affinity. (2) ________ ...
... Explain your answer. (2) _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ 7. Define electron affinity. (2) ________ ...
Electronic Structure of Atoms (i.e., Quantum Mechanics)
... This image shows a ring of 76 iron atoms on a copper (111) surface. Electrons on this surface form a two-dimensional electron gas and scatter from the iron atoms but are confined by boundary or "corral." The wave pattern in the interior is due to the density distribution of the trapped electrons.The ...
... This image shows a ring of 76 iron atoms on a copper (111) surface. Electrons on this surface form a two-dimensional electron gas and scatter from the iron atoms but are confined by boundary or "corral." The wave pattern in the interior is due to the density distribution of the trapped electrons.The ...
Document
... When you work the math… •the actual position of electrons can't really be specified •best we can do is say where they PROBABLY are •they are likely to be in cloud-like zones (called orbitals, not orbits) of varied shape •electrons with more energy can assume orbitals of increasingly bizzarre shape. ...
... When you work the math… •the actual position of electrons can't really be specified •best we can do is say where they PROBABLY are •they are likely to be in cloud-like zones (called orbitals, not orbits) of varied shape •electrons with more energy can assume orbitals of increasingly bizzarre shape. ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.