* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Catabolism of Carbon Skeletons of AAs1.06 MB
Clinical neurochemistry wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Proteolysis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Point mutation wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Butyric acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Metalloprotein wikipedia , lookup
Protein structure prediction wikipedia , lookup
Peptide synthesis wikipedia , lookup
Catalytic triad wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Genetic code wikipedia , lookup
Biochemistry wikipedia , lookup
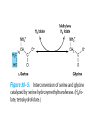
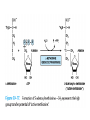
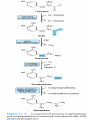
Catabolism of Carbon Skeletons of AAs Prof. Dr. Arzu SEVEN • The pathways of amino acid catabolism normally accounts for only 10-15% of human body's energy production. • 20 catabolic pathways converge to form only 6 major products, all of which enter citric acid cycle. • From there, C skeletons are diverted to gluconeogenesis or ketogenesis or are completely oxidized to CO2 and H2O . • Amino acids may be either glucogenic or ketogenic . • These amino acids that feed carbons into TCA cycle at the level of α-ketoglutarate, succinyl coA, fumarate or oxaloacetate and those that produce pyruvate ,can produce glucose via gluconeogenesis and are glucogenic (alanine, arginine, asparagine,aspartic acid, glycine, histidine, methionine, proline, serine, valine) • Those amino acids that feed carbons at the level of acetyl-coA or acetoacetyl coA are ketogenic (leucine, lysine) • Leucine is an exclusively ketogenic AA ,its degradation makes a substantial contribution to ketosis under starvation • Both glucogenic and ketogenic AAs isoleucine, phenylalanine, threonine, tryptophan, tyrosine • Amino acids that we can not synthesize are termed ESSENTİAL amino acids • Cysteine is not generally considered as an essential AA because it can be derived from non-essential amino acid serine, its sulfur must come from essential amino acid methionine. • Tyrosine is not required in the diet, but must be derived from essential amino acid phenylalanine. Conversion of AA to Specialized Products • Important products derived from AA include heme, purines, pyrimidines, hormones, neurotransmitters and biologically active peptides. Glycine • Water-soluble glycine conjugates: glycocholic acid and hippuric acid formed from food additive benzoate. • Drugs or drug metabolites with carboxyl groups are excreted in the urine as glycine conjugates. • Creatine and glutathione • Nitrogen and α-C of glycine are, incorporated into the pyrole rings and methylene bridge carbons of heme. • 4,5, and 7 atoms of purine glycine is degraded via 3 pathways: • Nonketotic hyperglycinemia: Defect in glycine cleavage enzyme activity • Glycine (serum) • mental deficiency • death in early childhood • At high levels glycine is an inhibitory neurotransmitter. O H O NH • Glycine Glyoxylate NAD NADHOxalate 2 2 D-Amino Acid oxidase 3 • Primary function of D-amino acid oxidase, present at high levels in the kidney, is to detoxify the ingested D-amino acids derived from bacterial cell walls and from grilled foodstuff. • Oxalate, from food or produced enzymatically in kidney, has medical significance as crystals of calcium oxalate in 75% of kidney stones. • (urolithiasis, nephrocalcinosis, early mortality from renal failure or hypertension) • Several enzyme cofactors play important roles in amino acid catabolism: Transamination requires pyridoxal phosphate • One Carbon transfer requires Biotin tetrahydrofolate and S-adenosylmethionine • Biotin transfers Carbon its most oxidized state (CO2) • Tetrahydrofolate transfers one carbon groups in intermediate oxidation states (as methyl groups) • s-adenosylmethionine transfers methyl groups (the most reduced state of carbon) Homocystinuria • A relatively rare autosomal recessive condition • Defect in methionine catabolism • Lack of an enzyme which catalyzes the transfer of sulfur from homocysteine to serine though the formation of cystathionine intermediate. • Mental retardation , vision problems, thrombotic strokes, coronary artery disease at young age. • Defective carrier-mediated transport of cystine results in cystinosis (cystine storage disease) with deposition of cystine crystals in tissues and early mortality from acute renal failure. • In cystinuria,a defect in renal reabsorption,cystine,lysine,arginine and ornithine are excreted. • The mixed disulfide of L-cysteine and Lhomocysteine,excreted by cystinuric patients,is more soluble and reduces formation of cystine calculi. • β-Alanine: • β-alanine, a metabolite of cysteine, is present in coenzyme A and as BalanyLdipeptides (carnosine, anserine ) • Cysteine: • A precursor of thioethanol amine portion of coenzyme A • A precusor of taurine that conjugates with bile acids such as taurocholic acid Histidine • Histidin Decarboxylation (-co2) Acid secretion İn stomach Histamine Allergic reaction vasodilatator • Arginine: • Formamidine donor for creatine synthesis • Precursor of nitric oxide, NO (neurotransmitter, smooth muscle relaxant and vasodilatator) • Phosphocreatine, derived from creatine, is an important energy buffer in skeletal muscle. • Creatine is synthesized from glycine, arginine. • Methionine, in the form of S_adenosylmethionine, acts as a methyl donor. • Tryptophan, lysine, phenylalanine, tyrosine, leucine, isoleucine and threonine acetyl coA and/or aceto acetyl -coA • Tryptophan: Nicotinamide Serotonin indolacetate • Principal normal urinary catabolites of tryptophan are 5-hydroxyindolacetate and indole-3-acetate. • Phenylalanine Tyrosine Dopamine NE E T3, T4 • Melanin is derived from tyrosine • Parkinson's disease is associated with underproduction of dopamine.It has traditonally been treated by L-Dopa administration. • Over production of dopamine in the brain may be linked to schizophrenia. • 5 hydroxytryptamine=serotonin: • A potent vasoconstrictor and stimulator of smooth muscle contraction. • Serotonin N-acetylation O-methylation melatonin 5 hydroxy indolacetate MAO Catalyzed oxidative deamination • Carcinoid(argentaffinoma) • Tm cells that over produce serotonin. • Glutamate decarboxylation gives rise to GABA, an inhibitory neurotransmitter • Its overproduction is associated with epilectic seizures. • GABA analogs are used in the treatment of epilepsy and hypertension. • γ-aminobutyrate (GABA) • Functions in the brain as an inhibitory neurotransmitter by altering transmembrane potential differences. • L-glutamate GABA decarboxylase • Branched Chain AA (leucine, valine, isoleucine) are oxidized as fuels primarily in muscle, adipose, kidney and brain tissue (extrahepatic tissues) • 1-Transamınation (branced-chain amino transferase (absent in liver) α- AA α- ketoacid • 2-Oxidative decarboxylation (by mitochondrial branched chain α-ketoacid dehydrogenase) • This multimeric enzyme complex resembles pyruvate dehydrogenase and α-ketoglutarate dehydrogenase being inactivated by phosphorylation and activated by dephosphrylation. • 5 cofactors (TPP, FAD, NAD, lipoate, coenzyme A) • 3-Dehydrogenation