* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Topic 16 Some non-metals and their compounds notes
Nucleophilic acyl substitution wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Stoichiometry wikipedia , lookup
Synthesis of carbon nanotubes wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Anoxic event wikipedia , lookup
Acid–base reaction wikipedia , lookup
Biochemistry wikipedia , lookup
Catalytic reforming wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Nitrogen dioxide poisoning wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Water splitting wikipedia , lookup
Photosynthesis wikipedia , lookup
Biosequestration wikipedia , lookup
Calcium looping wikipedia , lookup
Sulfur dioxide wikipedia , lookup
Sulfur cycle wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Electrolysis of water wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
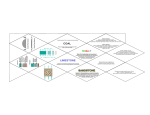
Hydrogen and Nitrogen Hydrogen is the most common element in the universe. It is a colourless, odourless gas which is less dense than air and extremely reactive. Unfortunately it is so light that there is none of it in our atmosphere. We can, however, produce hydrogen fairly easily by the electrolysis of water (H2O is broken down into the elements hydrogen and oxygen) or by the reaction of methane with steam. Nitrogen on the other hand makes up 78% of the air around us and can be obtained by fractional distillation of air or by removing the 21% oxygen from the air by combustion. Nitrogen is also a colourless, odourless gas which is very unreactive. Hydrogen and nitrogen can react together to make ammonia The Haber Process Air contains 78% nitrogen, and the nitrogen can be used to manufacture several important chemicals, including nitrogen-based fertilisers. Nitrogen-based fertilisers are important in agriculture for increasing the yields of crops. The starting compound for the manufacture of nitrogen-based fertilisers is ammonia, NH3. Ammonia can also be used to manufacture nitric acid. The process for the manufacture of ammonia was devised by the German physical chemist, Fritz Haber and was put into practice by the German chemical engineer, Carl Bosch. Haber Bosch The raw materials for the process are nitrogen and hydrogen. Nitrogen is obtained by the fractional distillation of liquid air. Hydrogen is obtained from the reaction of natural gas (methane) and steam. H2O(g) + CH4(g) 3H2(g) + CO(g) The purified gases are passed over a catalyst of iron at a high temperature and pressure. About 15% of the nitrogen and hydrogen react to produce ammonia. The gases are cooled to liquefy the ammonia, which is removed. Then the nitrogen and hydrogen are re-circulated through the reactor. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 1 The reaction between nitrogen and hydrogen is reversible, this means that ammonia also breaks down again into nitrogen and hydrogen. 3H2(g) + N2(g) 2NH3(g) H = -92 kJ.mol-1 Economics of the Haber Process The optimum conditions for this equilibrium reaction, which produce a reasonable yield of ammonia quickly, are decided as follows: Pressure An increase in pressure will shift the equilibrium to the right hand side, because this has fewer moles of gas. Therefore, the yield of ammonia will increase. In addition to its effect on the equilibrium position, increasing the pressure will: increase the reaction rate, establishing equilibrium faster. Since the molecules of gas are forced closer together, there will be more collisions in a given time. increase the cost. As pressure increases, the cost of industrial plant increases because, for example, vessels and pipes have to be thicker. A compromise has to be struck between increasing rate and yield, which are beneficial to the economics of the process, and increasing cost, which is not. In practice, a pressure of about 250 atmospheres is used. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 2 Temperature An increase in temperature will displace the equilibrium to the left hand side, because the backward reaction is endothermic. Therefore, the yield of ammonia will decrease. In addition to its effect on the equilibrium position, increasing the temperature will: increase the reaction rate, establishing equilibrium faster. Since the molecules of gas have more energy, a higher proportion will have energy greater than or equal to the activation energy and undergo successful collisions. increase the cost. Higher temperatures incur greater fuel costs. A compromise has to be struck between increasing rate, which is beneficial to the economics of the process, and increasing cost & decreasing yield, which are not. In practice, a temperature of about 450oC is used. Catalyst Addition of a catalyst has no effect on the equilibrium position and therefore does not affect the yield of ammonia. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 3 A catalyst provides an alternative reaction path with a lower activation energy. Therefore, it increases the reaction rate, establishing equilibrium faster. Using a catalyst leads to a small increase in cost. In practice, an iron catalyst containing promoters is used. USES OF AMMONIA Ammonia is a colourless gas with a very characteristic smell. It is an alkaline gas which turns damp red litmus paper blue. Manufacture of Fertilisers Ammonia is used in the production of fertilisers, which increase the yield of crops. The most common compounds used as fertilisers are: ammonium sulphate, which is made by the neutralisation of ammonia with sulfuric acid 2NH3 + H2SO4 (NH4)2SO4 ammonium nitrate, which is made by the neutralisation of ammonia with nitric acid NH3 + HNO3 NH4NO3 urea, which is made by the reaction of ammonia and carbon dioxide 2NH3 + CO2 CO(NH2)2 + H2O PROBLEMS WITH THE USE OF FERTILISERS Although artificial fertilisers have done much to increase world food production and reduce starvation, there are problems associated with their use. For example, the run-off of fertilisers from fields into water courses is a problem because: Too much fertiliser in water can lead to excessive algal growth (eutrophication). The algae form a thick mat on the surface of the water, which blocks out light and kills aquatic plants. This reduces oxygen levels in the water. When the mat starts to die and falls to the bottom, it removes oxygen from the water as it decomposes. As a consequence of this, fish are killed. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 4 The presence of nitrate in drinking water can cause health problems, particularly blue baby syndrome. In new-born babies, the presence of nitrate affects their ability to carry oxygen in the blood. Some changes to farming practice could reduce these problems. Farmers could, for example: not apply fertilisers when rain is expected, so that they are not washed into water courses before the plants have had a chance to absorb them apply fertilisers several times in small doses rather than in fewer larger doses Attempts are being made to reduce the need for artificial fertilisers by developing strains of the most important food crops which can fix their own nitrogen. Manufacture of Nitric Acid Nitric acid is made from ammonia in the Ostwald Process. Ammonia is oxidised by oxygen in the air in the presence of a hot platinum catalyst: 4NH3 + 5O2 4NO + 6H2O The nitrogen oxide (NO) formed in the above reaction is cooled and reacted with water and more oxygen to form nitric acid. 4NO + 2H2O + 3O2 4HNO3 Apart from its use in the manufacture of ammonium nitrate, nitric acid is also used in the manufacture of dyes, explosives and nylon IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 5 SULFUR You will see sulfur written as sulphur in textbooks – it is the same thing. Sulfur is a yellow non-metallic element. As an element it exits as small molecules of 8 sulfur atoms arranged in a ring. The molecule can be written as S8. Like carbon, which has two allotropic forms (diamond and graphite), sulfur has two allotropes called rhombic and monoclinic. Allotropes are different forms of the same element Most sulfur is obtained from compounds found in natural gas. Natural gas contains hydrogen sulfide, which smells of rotten eggs, 2H2S + O2 2S + 2H2O Sulfur can also be extracted from underground sulfur beds. In this case, hot water is pumped underground and the sulfur melts and is carried back to the surface. Sulfur is used to produce sulfuric acid though it can be used to toughen rubber tyres (vulcanizing), make drugs, cosmetics and even to modify the properties of concrete. Sulfur burns in air to produce sulfur dioxide which causes acid rain. Sulfur dioxide is a colourless gas with a choking smell S + O2 SO2 The SO2 produced dissolves in water (clouds) to for an acidic solution. SO2 + H2O H2SO3 Sulfur dioxide does, however, have its uses. It can be used to bleach wool and wood pulp for making paper. It also kills bacteria and can therefore be used to preserve food. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 6 THE MANUFACTURE OF SULFURIC ACID (The Contact Process) Concentrated sulfuric acid is manufactured from the raw materials sulfur, water and air. Step 1: Sulfur is burned in oxygen to form sulfur dioxide. S(s) + O2(g) SO2(g) H = -297 kJ/mol This reaction, which is exothermic, is a redox reaction. Sulfur is oxidized from an oxidation state of 0 to an oxidation state of +4. Step 2: The sulfur dioxide is then mixed with oxygen, and the mixture is passed over a catalyst of vanadium(V) oxide (V2O5) at a temperature of 450oC and a pressure of 2 atmospheres. This reaction, in which sulfur trioxide is formed, is exothermic. It is also a redox reaction, because sulfur is oxidized from an oxidation state of +4 to an oxidation state of +6. 2SO2(g) + O2(g) 2SO3(g) H = -192 kJ/mol The optimum conditions for this equilibrium reaction are decided as follows: Pressure An increase in pressure will shift the equilibrium to the right hand side, because this has fewer moles of gas. Therefore, the yield of sulfur(VI) oxide will increase. When pure oxygen is used, the volume reduction is from 3 volumes to 2. If air is used instead of oxygen, one volume of oxygen will be accompanied by 4 volumes of unreactive nitrogen. Therefore the volume reduction will be smaller (from 7 volumes to 6), and pressure will have a smaller effect on the equilibrium position. Even at low pressures, the equilibrium lies well to the right hand side, so the use of expensive high pressures is not justified. A compromise has to be struck between increasing rate and yield, which are beneficial to the economics of the process, and increasing cost, which is not. In practice, a pressure of about 2 atmospheres is used. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 7 Temperature An increase in temperature will displace the equilibrium to the left hand side, because the backward reaction is endothermic. Therefore, the yield of sulfur(VI) oxide will decrease. In addition to its effect on the equilibrium position, increasing the temperature will: increase the reaction rate increase the cost A compromise is needed between increasing rate, which helps the economics of the process, and increasing cost & decreasing yield, which do not. In practice, a temperature of about 450oC is used. Catalyst Addition of a catalyst has no effect on the equilibrium position and therefore does not affect the yield of sulfur(VI) oxide. Using a catalyst leads to a small increase in cost. In practice, a catalyst of vanadium(V) oxide is used. Step 3: The sulfur trioxide is dissolved in concentrated sulfuric acid to form fuming sulfuric acid (oleum, H2S2O7). Water is then carefully added to the oleum to produce concentrated sulfuric acid (98%). This reaction is very exothermic and has to be cooled by passing cold water through pipes. The heated water can then be used to generate electricity for the plant. USES OF SULFURIC ACID Sulfuric acid is used: as car battery acid in the manufacture of fertilizers in the manufacture of soaps & detergents Concentrated sulfuric acid is also a dehydrating agent– ie it can ‘remove water’ IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 8 Carbon The element carbon is found in the earth’s crust in two allotropic forms. Diamond is a clear, hard solid which does not conduct electricity, whilst graphite is a black, slippery solid which does conduct electricity. There are millions of compounds containing carbon. Most living things are made up of compounds containing carbon. Carbon cycle The carbon cycle describes the movement of carbon through the environment. Carbon exists in the ground, in the sea, in plants and animals, and in the air. The natural processes shown in the picture below can release carbon dioxide into the atmosphere (by combustion, decay and respiration) or remove carbon dioxide from the atmosphere (by dissolving in the sea and by photosynthesis. Carbon can also be "locked away" in the ground as a fossil fuel or by forming carbonate rocks. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 9 Carbon dioxide Carbon dioxide is released into the atmosphere when we burn fossil fuels or we thermally decompose carbonate rocks (see p12). Both of these processes take place daily on an industrial scale. CH4 + 2O2 CaCO3 2H2O + CO2 CaO + CO2 Carbon dioxide is heavier than air and does not support combustion. It is therefore used in fire extinguishers. Carbon monoxide Carbon monoxide (CO) is also produced when fuels burn in too little oxygen. We call this incomplete combustion. Carbon monoxide is an odourless, poisonous gas which binds to haemoglobin in blood cells preventing them from carrying oxygen around the body. Carbon monoxide poisoning therefore is very often fatal. CH4 + 1½ O2 2H2O + CO Carbonates You have already met decomposing carbonates in Topic 13 (behaviour of metals). Carbonates decompose when heated to form oxides and CO2. Only the carbonates of the most reactive metals (Na2CO3 and K2CO3) as so stable that they do not decompose when heated. Organic compounds Methane (CH4) is a colourless, odourless gas made up of the elements carbon and hydrogen. It is found trapped below impermeable rock under the sea floor where it formed millions of years ago. Some animals produce methane during the digestion of plant matter (cows and sheep) due to the presence of certain bacteria in their gut. Methane is also a greenhouse gas which contributes to global warming. There are millions of organic compounds such as proteins, carbohydrates, fats, pharmaceutical drugs, fuels and plastics which are all made of carbon bonded to other elements. You have already met these in Topics 17 & 18 (organic chemistry). IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 10 Global Warming Carbon dioxide, methane, water vapour and nitrous oxides absorb infra-red radiation in the same range that is given off by the Earth at night. More carbon dioxide in the atmosphere means more heat is absorbed, and less can escape at night into space. The overall effect is that the Earth receives more heat in the day than it can lose at night, so the temperature rises. The same thing happens in a greenhouse where glass instead of carbon dioxide is used to prevent the heat escaping at night. So, carbon dioxide, along with methane and water vapour have been called greenhouse gases. Climate change Carbon dioxide is causing the global temperature of the Earth to rise. This rise in temperature affects the climate all over the planet in ways which are hard to predict. Some areas may become wetter or dryer, other areas may become hotter. The main (predictable) directions of wind and ocean currents on the planet may slow down, speed up, or change direction. This could have catastrophic consequences for life on planet Earth, including rising sea levels because of ice melting at the poles and more extreme weather because of more convection in the hotter wetter atmosphere. Finally, some species may not be able to adapt to the different climate and may die out altogether. If we are causing global warming by burning fossil fuels we can do something about it. Cutting back on the burning of fossil fuels will help as will switching to more environmentally friendly sources of power such as wind, tidal and solar power. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 11 LIMESTONE Limestone is formed when sea creatures die and fall to the bottom of the sea floor. Over millions of years this material builds up and is pressed together to form limestone rock. These rocks are slowly pushed upwards and sea levels have fallen exposing the limestone. Limestone contains mostly calcium carbonate (CaCO3) which is an important raw material. Limestone is obtained by quarrying. Malham cove (limestone) Dover cliffs (chalk) marble quarry Thermal Decomposition When some substances are heated, they decompose (break down) into simpler substances. This process is called THERMAL DECOMPOSITION. Calcium carbonate undergoes thermal decomposition when heated in a kiln to about 1100 oC, forming calcium oxide (quicklime) and carbon dioxide. Other metal carbonates decompose in a similar way. The lower the metal is in the reactivity series, the lower the temperature at which this decomposition takes place. CaCO3(s) CaO(s) + CO2(g) Hydration Quicklime reacts with water in a very exothermic reaction, forming calcium hydroxide (slaked lime). CaO(s) + H2O(l) Ca(OH)2(s) The Limestone Cycle H2O LIMESTONE (calcium carbonate) CO2 CO2 QUICKLIME (calcium oxide) SLAKED LIME (calcium hydroxide) IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS H2O 12 Uses of Calcium Carbonate & Calcium Hydroxide Building Material Limestone is used as a building material. A form of limestone, called Portland stone, was used in the construction of St Paul’s Cathedral in London. Limestone is a hard rock, but it erodes badly in polluted urban environments, because it dissolves readily in acids. Marble is used in sculpture and as a building material. St. Paul’s Cathedral marble bust Removal of Acidity Powdered limestone is used to neutralise acidity in lakes and in light, sandy soils. Slaked lime (calcium hydroxide) is used to neutralise acidity in heavy soils and in drinking water. Cement Manufacture Cement is made by roasting powdered limestone powdered clay gypsum in a rotary kiln. Cement is then used to make concrete. Flue Gas Desulfurisation is a process used to prevent SO2 escaping into the atmosphere. Waste gases containing SO2 are passed through a flue (chimney) containing calcium hydroxide (Ca(OH)2) which absorbs the SO2 producing calcium sulphite (CaSO3) and water. Ca(OH) 2 + SO2 CaSO3 + H2O This can easily be oxidised to to make hydrated calcium sulphate (CaSO 4. 2H2O), also known as gypsum, which is used to make the cement and also plasterboard for the building industry. 2CaSO3 + O2 + IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 4H2O CaSO4. 2H2O (gypsum) 13 Glass Manufacture Glass is made by heating a mixture of: limestone sand sodium carbonate Iron production Limestone is added to the Blast Furnace, in the production of iron, to remove acidic impurities present in iron ore. IGCSE TOPIC 16: NON METALS & THEIR COMPUNDS 14 NEUTRALISATION GLASS heavy soils drinking water SLAKED LIME lakes light soils add water QUICKLIME heat + sand & sodium carbonate heat LIMESTONE CHALK MARBLE CALCIUM CARBONATE heat + powdered clay BUILDING removal of impurities CEMENT IRON & STEEL TOPIC 11.16: Non-metals and their compounds 15 CONCRETE mix with water sand crushed rock Topic 16 Non-metals and their compounds Summary questions 1 2 3 TOPIC 11.16: Non-metals and their compounds 16 4 5 TOPIC 11.16: Non-metals and their compounds 17 6 7 8 TOPIC 11.16: Non-metals and their compounds 18 9 TOPIC 11.16: Non-metals and their compounds 19 10 TOPIC 11.16: Non-metals and their compounds 20 (d) 11 TOPIC 11.16: Non-metals and their compounds 21 12 TOPIC 11.16: Non-metals and their compounds 22 TOPIC 11.16: Non-metals and their compounds 23