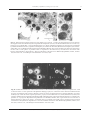
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Paralytic Shellfish Poisoning Toxins: Biochemistry and Origin Masaaki Kodama
Survey
Document related concepts
Transcript
Aqua-BioScience Monographs Vol. 3, No. 1, pp. 1–38 (2010) www.terrapub.co.jp/onlinemonographs/absm/ Paralytic Shellfish Poisoning Toxins: Biochemistry and Origin Masaaki Kodama Laboratory of Marine Biochemistry, Department of Aquatic Biosciences, Graduate School of Agricultural and Life Sciences, The University of Tokyo 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657, Japan e-mail: [email protected] Abstract Plankton feeders such as bivalves often become toxic. Human consumption of the toxic bivalve causes severe food poisoning, including paralytic shellfish poisoning (PSP) which is the most dangerous because of the acuteness of the symptoms, high fatality and wide distribution throughout the world. Accumulation of PSP toxins in shellfish has posed serious problems to public health and fisheries industry. The causative organisms of PSP toxins are known to be species of dinoflagellates including those belonging to the genus Alexandrium, Gymnodinium catenatum and Pyrodinium bahamense var. compressum. Bivalves accumulate PSP toxins during a bloom of these dinoflagellates. Thus, the dinoflagellate toxins have been considered as being concentrated in bivalves through food web transfer. However, field studies on the toxin level of bivalves in association with the abundance of toxic dinoflagellates could not support the idea. A kinetics study on toxins by feeding experiments of cultured dinoflagellate cells to bivalves also showed similar results, indicating that toxin accumulation of bivalves is not caused by simple accumulation of toxins due to food-web transfer. Based on the discovery of toxin-producing bacterium in the cells of toxic dinoflagellates, this study suggests that PSP toxins in toxic dinoflagellates are catabolites of bacterial substance in dinoflagellate cells. On the other hand, tetrodotoxin (TTX), a puffer toxin, is reported to be produced by some species of bacteria. Thus, the origin of TTX of puffer is considered to be bacteria. However, the mechanism for puffer to possess TTX through bacteria is unknown. The present study revealed that organisms bearing either TTX or PSP toxins possess both toxins, although the proportion of both is different among the specimens. In fact, a significant level of TTX is detected in A. tamarense. In addition, TTX-like toxin-producing bacteria are found to be infected in the liver of toxic specimens of puffer. These findings strongly suggest that a similar mechanism between bacteria and toxic organisms is involved in the production of TTX and/or PSP toxins in toxic organisms. 1. Introduction Saxitoxin (STX) and its analogs (STXs) are potent neurotoxins that block voltage-gated sodium channels on excitable cells (Kao 1966). These toxins are produced by several species of dinoflagellates such as Alexandrium tamarense and A. catenella (Schantz 1986). During a bloom of these species, filter-feeding bivalves become toxic by ingesting them. Human consumption of toxic shellfish results in paralytic shellfish poisoning (PSP) owing to its main symptom, paralysis. Bivalve farming areas infested by these species run costly monitoring programs to check for the shellfish toxicity and the causative dinoflagellates in the water. When these are present, regular tests for toxins in associated seafood products such © 2010 TERRAPUB, Tokyo. All rights reserved. doi:10.5047/absm.2010.00301.0001 Received on September 8, 2009 Accepted on October 13, 2009 Published online on April 9, 2010 Keywords • paralytic shellfish poisoning toxins • saxitoxin • gonyautoxin • tetrodotoxin • dinoflagellate • Alexandrium tamarense • bivalve • intracellular bacteria • bacterial infection as bivalves are carried out and often result in the prohibition of harvesting these products. Thus, the contamination of bivalves with STXs has posed severe problems for the bivalve culture industries as well as for public health. Many studies have been carried out on the toxinproducing dinoflagellates and the toxins they produce and there is much known about their taxonomy and ecology (Steidinger 1993). The chemical structures and pharmacological properties of the toxins have been well defined (Schantz 1986; Kao 1966). On the biosynthesis of the toxin, Shimizu et al. (1984) proved that the skeleton of STXs is synthesized from arginine and acetate based on the tracer experiments using PSP-producing cyanobacterium Aphanizomenon flosaque, and proposed the unique biosynthetic pathway. Very recently, this pathway has been 2 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 1. Structures of PSP toxins proved to be true with slight modification through chemical and biochemical studies (Kellmann et al. 2008a, b). However, these data are also obtained from toxinproducing cyanobacteria. Currently there is no information on the biosynthesis of PSP toxins in toxin-producing dinoflagellates, although PSP toxins have been detected in bacteria isolated from toxin-producing dinoflagellates (Kodama et al. 1988; Doucette and Trick 1995; Gallacher et al. 1997). Studies on the role of toxin-producing bacteria in the marine ecosystem are considered to be becoming more important. 2. PSP toxins found in bivalves and dinoflagellates STX and its derivatives belong to a group of neurotoxins which block voltage-gated sodium channels on excitable cells (Kao 1966). These toxins are known to be produced by several species of dinoflagellates such as Alexandrium tamarense and A. catenella (Schantz 1986). During a bloom of these species, bivalves, filter-feeding mollusks, accumulate toxins by ingesting them. The PSP toxins consist of a group of toxin components, the structures of which are similar to each other. These compounds are water soluble alkaloids. A typical toxin is STX which was first isolated from the toxic Alaskan butter clam Saxidomus giganteus (Schantz et al. 1957). As STX is highly hygroscopic and hard to crystallize, extended efforts were required to elucidate its chemical structure. Finally, Schantz et al. (1975) succeeded in determining its chemical structure, which possesses a characteristic doi:10.5047/absm.2010.00301.0001 tricyclic ring system with two guanidium moieties and a hydrated ketone. Subsequently, various derivatives of STX, such as neoSTX and gonyautoxin (GTX) 1–4, and B and C toxins, have been isolated from contaminated shellfish as well as the causative dinoflagellates, and their chemical structures have been identified (Oshima et al. 1977; Shimizu et al. 1978; Hall et al. 1980; Kobayashi and Shimizu 1981; Shimizu and Hsu 1981; Wichmann et al. 1981; Harada et al. 1982; Koehn et al. 1982). Currently, more than 20 derivatives are known (Oshima 1995a). The toxin in shellfish and toxic dinoflagellates is usually a mixture of two or more toxin components. Among them, STX is rather a minor component. Figure 1 shows the structures of the components commonly found in toxin-contaminated shellfish and the causative dinoflagellates. All of these are derivatives of STX. As shown in Fig. 1, the toxins can be classified into two categories: N1-H type and N1-OH type groups. The simplest component of the former group is STX, and that of the latter is neoSTX. Another structural variation in both groups is the presence or absence of an O-sulfate at C11 position and an N-sulfate on the carbamoyl groups. These variations greatly influence the physical and chemical characters as well as the pharmacological characters. Generally, the toxins are considered to be water soluble and heat stable. The stability varies greatly, depending on the pH and the structures (Shimizu 2000). At an alkaline pH, all the toxin components degrade quickly, even at room temperature. Generally the toxins are stable under acidic conditions and it is noteworthy that the po© 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 3 Table 1. Specific toxicity of each toxin component (MU·µmol–1)*. Component Specific toxicity Component Specific toxicity STX neoSTX dcSTX GTX1 GTX2 GTX3 GTX4 2483 2295 1274 2468 892 1584 1803 dcGTX2 dcGTX3 GTX5 C1 C2 C3 C4 1617 1872 160 15 239 33 143 *MU: mouse unit; one MU is a dose of toxin which kills one 20-g mouse (ddY strain) in 15 min. According to Oshima, Y. Post-column derivatization HPLC methods for paralytic shellfish poisons. In: Hallegraeff GM, Anderson DM, Cembella AD (eds). Manual on Harmful Marine Microalgae. IOC Manuals and Guides No. 33. Intergovernmental Oceanographic Commission, UNESCO, Paris. 1995; pp. 81–111. tency of a toxin is different among toxin components. Table 1 shows the specific toxicity of each toxin component expressed as mouse units (MU) per µm mole of toxin, in which 1 MU is a dose of toxin sufficient to kill a male mouse (ddY strain) in 15 min (Oshima 1995a). 3. Accumulation of paralytic shellfish poisoningtoxins in bivalve during a bloom of toxic dinoflagellates in the field PSP is a dangerous food poison because of its high fatality, acuteness in developing symptoms, and worldwide occurrence. It is confirmed by many authors that bivalves become toxic during blooms of toxic dinoflagellates. PSP has long been known along the Pacific and Atlantic coasts of North America and Canada, and many fatal cases have been recorded (Prakash et al. 1967). Poisoning occurred in September of 1972 and in June and September of 1974 after large-scale red tides of A. tamarense occurred along the East Coast, and 26 persons were intoxicated during the 1972 incidence. It was prohibited to take shellfish along a 3200 km coastline, which resulted in great economic loss (LoCicero 1975). In Japan, PSP had not been recognized before the incident at Toyohashi in 1948 (Hashimoto et al. 1950). Since then, a few cases of PSP have been observed and so detailed surveys on the causative dinoflagellates and bivalve toxicity have not been conducted. Ofunato Bay is one of the places where PSP due to consumption of scallop Chlamys nipponensis akazara occurred in 20 people including one fatal case in 1961 (Kawabata et al. 1962). The toxin in toxic shellfishes, as well as possibly causative organisms in the bay, has been examined by some investigators (Murano 1975), though not satisfactorily. After the 1977 incident, Fukuyo (1979) isolated an Alexandrium species from the bay, and identified it as Gonyaulax excavate (Alexandrium tamarense). Oshima and Yasumoto (1979) showed that the toxin from cultured A. tamarense consisted of GTX1, 2, 3, 4 and neosaxitoxin doi:10.5047/absm.2010.00301.0001 (neoSTX). Fukuyo (1979) observed that toxic dinoflagellates in Ofunato Bay presented three peaks in abundance in 1979. They found that the first and second peaks appearing in spring and summer, respectively, consisted of Alexandrium tamarense (former Protogonyaulax tamarensis) and the third peak in autumn consisted of A. catenella (former P. catenella). In order to make clear any possible relationship between the appearance of toxic dinoflagellates and the toxification of the scallop feeding on those plankton, the occurrence of Alexandrium spp. in Ofunato Bay was monitored in association with the toxin level of scallop Patinopecten yessoensis in Ofunato Bay (Ogata et al. 1982). Seawater samples for monitoring the cell density of A. tamarense were collected, usually twice a week, from different depths of water (2 m-intervals) at Shizu (St. S. 24 m depth) and Yamaguchi (St. Y. 14 m depth) in Ofunato Bay (Fig. 2). Some 500 mL of seawater was concentrated by gravity filtration using a membrane filter. The concentrate was made up to 20 to 40 mL and each 1 mL portion was used for microscopic counting of Alexandrium cells. When the population of Alexandrium tended to increase, seawater samples were collected almost every day. Nontoxic scallop Patinopecten yessoensis were transplanted from Mutsu Bay, Aomori Prefecture, to the two stations at a 10 m depth. Five scallop specimens were collected for testing for toxicity whenever the seawater sampling was done. Digestive glands were excised from them, combined and measured for PSP toxicity by the AOAC method (Horowitz 1984). Alexandrium appeared twice in spring and again in autumn in this bay. Similar results were obtained from both stations. The results from St. S in Fig. 3 show that relatively small number of Alexandrium began to appear in February and March, and disappeared in April at both stations. The plankton appeared again in May and sharply increased its density, showing large fluctuations. It diminished by the end of June at both stations. Alexandrium appeared again in September and reached maximum © 2010 TERRAPUB, Tokyo. All rights reserved. 4 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 2. Map of Ofunato Bay showing two sampling station. Reprinted with permission from Bulletin of the Japanese Society of Scientific Fisheries, 48, Ogata et al., The occurrence of Protogonyaulax spp. in Ofunato Bay, in association with the toxification of the scallop Patinopecten yessoensis. 563–566, Fig. 1, © 1982, the Japanese Society of Fisheries Science. abundance in mid-October at both stations. It disappeared by the end of October, showing that it appeared three times in 1980. The Alexandrium which occurred in spring and summer was identified as A. tamarense while that occurred in autumn was identified as A. catenella. In a continuous survey in the bay over 10 years, similar patterns were observed. Toxicity of scallop increased in association with the occurrence of Alexandrium. However, the ratio of the toxicity level to the cell number significantly varied, even in the same species. The ratio for the spring A. tamarense was generally larger than for that of summer, suggesting that both organsms differed substantially from each other in the amount of toxin per cell. It has been reported that the amount of toxin contained in a cultured A. tamarense cell varied depending upon growth conditions (Ogata et al. 1987a). The toxin contents of natural cells in the field seemed to reflect their physiological conditions. Once intoxicated, scallop did not become nontoxic in July and August, even during several months after the responsible plankton disappeared. They still maintained considerable amounts of toxin, indicating that the toxin can not be eliminated easily from doi:10.5047/absm.2010.00301.0001 Fig. 3. Seasonal changes in cell number of Alexandrium (—) and in toxicity level of scallop (---) at St. Shizu of Ofunato Bay. Reprinted with permission from Bulletin of the Japanese Society of Scientific Fisheries, 48, Ogata et al., The occurrence of Protogonyaulax spp. in Ofunato Bay, in association with the toxification of the scallop Patinopecten yessoensis. 563–566, Fig. 3, © 1982, the Japanese Society of Fisheries Science. the scallop. As shown in Fig. 4, it has been confirmed by the toxicity change of highly toxic scallop which was reared in an aquarium (Oshima et al. 1982a). When the highly toxic scallop was reared in Alexandrium-free water, around two thirds of toxin was lost within 1 week. However, a considerable amount of toxin was maintained for a long period. As described above, the species appearing in spring was A. tamarense while that in autumn was A. catenella. The scallop toxicity was correlated with the abundance of A. tamarense (Fig. 4), whereas the association of A. catenella with shellfish toxicity was not clear in this bay. Interestingly, some curious phenomena were observed during the monitoring. One of them is that there was no marked toxicity increase in the shellfish when A. tamarense reached the maximum abundance. The toxicity of scallop increased to the maximum, then the population of A. tamarense was decreasing. The timelag between the two maxima was about one week. Another is that the scallop showed a repeated rise and fall in toxicity during the period, when the supply of the toxin seemed to be apparently suppressed. These apparently © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 5 ins due to exposure to toxic bloom of dinoflagellate, become toxic because of ingestion of toxic feces from toxic scallop (Kikuchi et al. 1996). These facts show that scallop possibly accumulate toxins from toxic feces of the toxic scallop as well as from toxic dinoflagellates. Thus, it seems to be difficult to understand the kinetics of toxins accumulated in scallop from dinoflagellates from the data on field survey. 4. Toxin production of Alexandrium species Fig. 4. Decline of the toxicities of scallop Patinopecten yessoensis (a) and mussel Mytilus galloprovincialis (b) in laboratory tanks. Reprinted with permission from Bulletin of the Japanese Society of Scientific Fisheries, 48, Oshima et al., Features of paralytic shellfish poison occurring in Tohoku district. 525–530, Fig. 4, © 1982, the Japanese Society of Fisheries Science. curious phenomena may be due to the difference of the toxin content of the cells under different environmental and/or physiological conditions. Accurate analysis of the parameters such as toxin contents of the moving plankton cells is actually difficult. Thus the one week time-lag observed between the peaks of shellfish toxicity and abundance of toxic dinoflagellate may be within the limit allowed usually in the fixed point observation on the sea. However, similar phenomena have been observed almost every year in the monitoring in Ofunato Bay (Sekiguchi et al. 1989) and in Okirai Bay in the same prefecture using mussel Mytilus galloprovincialis as a monitoring bivalve (Oshima et al. 1982a). This phenomenon is difficult to explain if the toxins in the dinoflagellates transfer to the shellfish via the food chain. It is hard to evaluate the exchange of the toxin amount between dinoflagellates and shellfish in a field survey in which samples of shellfish and plankton are collected periodically at a station set in the field, even though the frequency of the sampling is increased. In addition, it is pointed out that nontoxic scallop introduced to the scallop culture area where scallop accumulated toxdoi:10.5047/absm.2010.00301.0001 Toxin production of the toxic dinoflagellate has been indicated to be affected by a variety of physiological or environmental factors: the toxicity of A. tamarense is different in its various growth stages (Prakash 1967; White and Maranda 1978; Oshima and Yasumoto 1979; Singh et al. 1982; Boyer et al. 1985); salinity affects the toxin production of A. tamarense (White 1986); toxin production of A. tamarense changes depending on the different growth stage of the cells (Kodama et al. 1982a). Here we describe the effect of water temperature and light intensity on growth rate and toxicity change in A. tamarense. A clonal culture was obtained from a single vegetative cell of A. tamarense isolated from Ofunato Bay, Iwate Prefecture, Japan in March, 1984, when the species was blooming. The clone obtained was mass-cultured and maintained in enriched seawater T1 medium (Ogata et al. 1987b) at 15°C under an illumination of 3000 lux with an LD cycle of 16 h:8 h. This clone was cultured under different temperatures and light intensities after acclimation of the clonal population during a period of 20 days. An aliquot of the cells from each culture was taken every day to count the cell density under a microscope. The growth rate (µ2 was estimated from cell counts in the exponential phase according to Fukazawa et al. (1980). The growth rate decreased with the decrease of water temperature and light intensity. The toxicity of the cell increased with a decrease of growth rate (Fig. 5). Interestingly, this growth rate was decreased by lowering the temperature rather than by lowering the light intensity. This fact coincided well with our observation that the toxicity of A. tamarense at a lower temperature was higher than that at a higher temperature (Ogata et al. 1987a). This fact suggests that photosynthesis is necessary for the production of toxin in A. tamarense. In order to confirm the effect of light on the toxin production of A. tamarense, we inhibited growth by lowering either water temperature or light intensity during mid-exponential phase, and then examined the toxicity of the cells before and after treatment. As shown in Fig. 6, growth stopped when either temperature or light intensity was lowered. After this treatment, toxicity of the cells (the growth of which was slowed by lowering temperature) increased, while that by lowering light intensity did not (see Table 2). These facts indicate that photosynthesis is © 2010 TERRAPUB, Tokyo. All rights reserved. 6 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 5. Alexandrium tamarense. Relation between toxicity and growth rates measured in different water temperature (a) and light intensity (b). Different toxicity formulas are used in A and B. Reprinted with kind permission from Springer Science + Business Media: Marine Biology, Effect of water temperature and light intensity on growth rate and toxicity change in Protogonyaulax tamarensis, 95, 1987, 217–220, Ogata, Ishimaru and Kodama, Fig. 3. Glover et al. (1975) showed that amino acids were produced during short periods of photo-assimilation. We added NO3 as a nitrogen source in the culture experiments. Therefore, de novo synthesis of amino acids should be carried out utilizing incorporated NO3. The NO3 assimilation of microalgae is reported to be light-dependent (Morriss 1974; Syrett 1981), and this was also confirmed in A. tamarense (MacIsaac et al. 1979). Therefore, a decrease of light intensity at mid-exponential phase of our experiments seems to significantly retard de novo synthesis of amino acids by suppressed nitrogen assimilation. The interruption of toxin production observed during the light retardation at the mid-exponential phase seems to depend on suppressed de novo synthesis of amino acids because of the decrease of NO3 assimilation. From these, de novo synthesis of amino acids by photo-assimilation is thought to play an important role in the toxin production of A. tamarense. 5. Transformation of toxins in dinoflagellates and bivalves Fig. 6. Alexandrium tamarense. Growth curves obtained by lowering the temperature (a) and light intensity (b) in the midexponential phase. Reprinted with kind permission from Springer Science + Business Media: Marine Biology, Effect of water temperature and light intensity on growth rate and toxicity change in Protogonyaulax tamarensis, 95, 1987, 217–220, Ogata, Ishimaru and Kodama, Fig. 5. necessary for the toxin production of A. tamarense. They also explain well the observation of the field survey that the toxin content of A. tamarense occurred in March and is higher than that occurred in May to June (Ogata et al. 1982). Shimizu et al. (1984) showed that STX analogs were synthesized from arginine and acetic acid in A. tamarense. doi:10.5047/absm.2010.00301.0001 PSP toxins have been detected in several species of Alexandrium, Gymnodinium catenatum and Pyrodinium bahamense var. compressum. These species are photosynthetic dinoflagellates. Thus, PSP toxins are considered to be produced by these dinoflagellates. This view is supported by the fact that toxin production has been confirmed in axenic cultures of these species (Burke et al. 1960; Singh et al. 1982). That metabolism of toxins, including biosynthesis, occurs in the cells of these dinoflagellates was indicated by these experiments. Various toxin components found in the toxic species of dinoflagellates could be metabolites of toxin metabolism that occurred in the dinoflagellates. Interestingly, the toxin profile of cultured cells does not change significantly under different culture conditions such as different temperatures, light intensities, and levels of nutrients, if the culture is monoclonal (Boyer et al. 1986, 1987; Cembella et al. 1987; Ogata et al. 1987b). Therefore, the toxin profiles of dinoflagellates isolated from various areas have been elucidated by many research groups. These studies indicate that the toxin profile is often specific to a species of dinoflagellates. For example, G. catenatum shows a characteristic toxin profile which consists of N-sulfocarbamoyl toxins such as B and C toxins as major components (Oshima et al. 1990). GTXs are rarely detected in the species. On the other hand, P. bahamense var. compressum contains B toxins and their carbamate counterparts, STX and neoSTX, as dominant toxins. A significant amount of decarbamoyl saxitoxin (dcSTX) (see the structure in Fig. 1) is also found in this species (Harada et al. 1982, 1983). A difference in toxin profile has also been observed among species belonging to the genus Alexandrium. C2 and GTX4 are the dominant © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 7 Table 2. Alexandrium tamarense (OF84423D3). Effect of slowdown of the growth rate on toxicity. Treatment Harvesting Toxicity Total toxin concentrationa MU/104 cell MU/mm3 (nmol/104 cells) Ab Before treatment After treatment 0.68 1.63 2.30 4.84 0.491 1.007 Bc Before treatment After treatment 0.64 0.68 2.71 3.13 0.398 0.494 Calculated from the toxin composition Treatment A: Growth rate was slowed by lowering temperature at the mid-exponential phase c Treatment B: Growth rate was slowed by lowering light intensity at the mid-exponential phase Reproduced with kind permission from Springer Science + Business Media: Marine Biology, Effect of water temperature and light intensity on growth rate and toxicity change in Protogonyaulax tamarensis, 95, 1987, 217–220, Ogata et al., Table 3. a b components in Alexandrium (Protogonyaulax) tamarense and A. catenella (Oshima et al. 1990), while GTX1–4 are dominantly found in A. tamiyavanichii (Kodama et al. 1988; Ogata et al. 1990). This species was isolated from the Gulf of Thailand and identified as A. cohorticula, but it was reclassified as a new species A. tamiyavanichii by Balech: A. ostenfeldii showed a toxin profile consisting of B toxins and their carbamate counterparts as dominant toxins (Hansen et al. 1992). Strains of the same species isolated from different geographical areas often show the different toxin profile. Oshima et al. (1993a) showed that a Tasmanian isolate of G. catenatum contains C4, which was absent in the same species isolated from other areas (Ikeda et al. 1989). Furthermore, they detected novel components, 13-deoxydecarbamoyl derivatives, in some strains of G. catenatum (Oshima et al. 1993b). A similar phenomenon was also observed among different strains of A. minutum. Most of the isolate of A. minutum from various areas show a characteristic toxin profile consisting of GTX1–4 (Oshima et al. 1989; Franco et al. 1994). In contrast, neoSTX, STX, and C toxins were detected in an isolate from New Zealand (Chang et al. 1997). Another, similar phenomenon was observed among clones of A. tamarense (=Protogonyaulax tamarensis) isolated from different areas of Japan (Oshima et al. 1982b). It is noteworthy that nontoxic strains are often found in toxic species. Some strains of A. tamarense are reported to be nontoxic (Loeblich and Loeblich III 1975). Oshima et al. (1993b) also reported that a clonal culture of G. catenatum established from a cyst isolated from Tasmania is nontoxic. These facts may indicate that PSP toxins are not essential for the survival of these dinoflagellates. Although little is known about the metabolism of toxins in dinoflagellates, some enzymes involved in toxin transformation have been reported. Oshima (1995b) showed the presence of oxidase activity doi:10.5047/absm.2010.00301.0001 in some clones of A. tamarense that catalyzes the oxidation of N1-H of GTX2,3 to form GTX1,4. However, this enzyme is not always present in all the isolates of A. tamarense. On the other hand, N-sulfotransferase that converts carbamate toxins to the corresponding C toxins has been detected in G. catenatum (Oshima 1995b; Yoshida et al. 1996). The occurrence of these enzymes may explain the characteristic toxin profile of some dinoflagellate species. The hereditary character of the toxin profile in dinoflagellates supports the role of enzymes in the characteristic toxin profile of dinoflagellates. Various toxin components found in the dinoflagellates may be metabolites derived from a de novo product in the biosynthesis of the STX skeleton, though so far this is not known with certainty. It is plausible that enzymes are involved in the transformation of the de novo toxin to various toxin components. The profile of the toxins accumulated in shellfish during a bloom of toxic dinoflagellates reflects that of the causative dinoflagellate. However, the toxin components accumulated in the shellfish change after the causative dinoflagellates disappear from the environment (Oshima 1995a). The shellfish themselves are not involved in biosynthesis of the toxins, and thus the toxin components transferred from the dinoflagellates undergo metabolic transformation in the shellfish. In an earlier study, Shimizu and Yoshioka (1981) showed that transformation of PSP toxins occurs in the shellfish. They incubated an homogenate of the scallop Patinopecten magellanicus contaminated with neoSTX and GTX2,3, and found that the proportion of STX increased after incubation. These facts showed that neoSTX and GTXs are converted to STX in the shellfish. From these data, they suggested the presence of enzymes in the shellfish which are involved in the transformation of toxin components, though little is known about such enzymes. The rate of the transformation is very low indicating that enzyme © 2010 TERRAPUB, Tokyo. All rights reserved. 8 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 activity in the scallop is low, if the reaction is enzymatic. Oshima (1995b) screened the enzyme activities which transform C toxins in the shellfish, and detected the activity to hydrolyze N-sulfocarbamoyl of C toxins to form dcGTXs in two species of clams, Mactra chinensis and Peronidia venulosa. Enzyme activity to hydrolyze carbamate toxins was also detected in some species of shellfish (Sullivan et al. 1983). However, the enzyme involved in the transformation of GTXs to STX has not been found in shellfish, though this transformation is observed generally in the shellfish. 6. Non-enzymatic transformation of PSP toxins: formation of unique intermediate conjugates of PSP toxins and thiols As described above, GTXs accumulated in shellfish are gradually transformed to STXs. The rate of transformation is very slow (Oshima 1995a), suggesting that the reaction is not enzymatic. On the other hand, Kotaki et al. (1985) reported that a part of GTXs incubated with bacterial cells are were transformed to STXs, showing that these bacteria possess an ability to transform GTXs to STXs. They also indicated that the factors involved in toxin transformation occur widely in the ecosystem. Thus, we incubated GTXs in the water-soluble extract of a bacterium Moraxella sp. (PTB-1) (Sakamoto et al. 2000) and found that the extract prepared with 0.1 M phosphate buffer (pH 7.4) often converted GTXs to STXs. However, this transformation activity was not always observed, indicating that the activity of the extract is not stable. However, once the activity was obtained from the bacterium, it is very stable, even after heat treatment. These facts indicate that the activity is not due to the enzyme. On the other hand, Asakawa et al. (1987) reported that glutathinone (GSH), a biological reductant widely occurring in organisms, transform GTX1, 2, 3 to STX. Bacteria are known to possess a membrane-bound enzyme that degradates GSH (Nakayama et al. 1984). If the activity is due to GSH, a part of GSH seems to be degradated by the enzyme during preparation of water-soluble extract of the bacterium. Thus, the GSH levels in the extracts with and without L -serine, the inhibitor of GSH degradating enzyme, were analyzed. At the same time, transformation of GTXs to STXs was also analyzed (Sakamoto et al. 2000). Figure 7 shows the change of GSH level in the extracts prepared from the homogenates with and without the inhibitor. The GSH level in the extract in the absence of the inhibitor was 0.21 mM when the extract was prepared just after the cells were homogenated (Fig. 7, upper). However, this level decreased gradually when the homogenate was incubated at 30°C. After 24 h incubation, the level was decreased to 0.06 mM. In contrast, GSH levels in the extract in the presence of the inhibitor were 0.31 mM when the extract was prepared just after doi:10.5047/absm.2010.00301.0001 Fig. 7. Change of toxin components and glutathione (GSH) in the mixture of gonyautoxins (GTX) 2,3 and bacterial homogenates during incubation. GTX2,3 (10 µmol·L–1) was incubated with the homogenates at 30°C. Change of toxin components in the homogenate with 0.1 M phosphate buffer, pH 7.4. (b) Change of toxin components in the homogenate with 0.1 M borate buffer, pH 7.4 containing 100 mM L -serine. 䊊, GTX2,3; 䊉, saxitoxin; white bar, GSH level. PSP, paralytic shellfish toxins. Reprinted from Sakamoto et al., Formation of intermediate conjugates in the reductive transformation of gonyautoxins to saxitoxins by thiol compounds. Reprinted with permission from Fisheries Science, 66, Oshima et al., Features of paralytic shellfish poison occurring in Tohoku district. 136–141, Fig. 2, © 2000, the Japanese Society of Fisheries Science. the cells were homogenized (Fig. 7, lower). This level also decreased slightly, but 0.21 mM of GSH remained in the extract, even when the homogenate was incubated for 24 h. These results show that GSH in bacterial cells is easily decomposed by the GSH degradating enzyme during extraction and incubation, unless the extract was prepared in the presence of the inhibitor against the enzyme (Sakamoto et al. 2000). Figure 7 also shows the change of GTX2,3 added to these homogenates during incubation. When GTX2,3 was incubated with the cell homogenate in the presence of the inhibitor, more than 80% of GTX2,3 disappeared within 0.5 h, although no STX was detected (Fig. 7, lower). After 1 h incubation, most of GTX2,3 disappeared and a trace amount of STX appeared. The level of STX increased gradually during further incubation and reached 2.2 mM after 24 h incubation, showing that about 20% of GTX2,3 added to the homogenate was transformed to STX. This phenomenon also suggests the formation of an intermediate compound which cannot be detected by © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 HPLC in the transformation reaction of GTX2,3 to STX (Sakamoto et al. 2000). When GTX2,3 was added to the homogenate without the inhibitor, about 20% of toxins also disappeared within 0.5 h (Fig. 7, upper). However, the level of GTX2,3 did not change during further incubation. No STX nor other known PSP toxin components were detected during incubation. These results show that the bacterial activity to transform GTX2,3 to STX is mainly due to GSH. Unstable activity of bacterial extract prepared in the absence of the inhibitor seems to be caused by degradation of GSH by the GSH degradating enzyme in the bacterial homogenate during extraction. Membrane-bound protein fraction prepared by 2-mercaptoethanol (ME) containing a buffer also showed a significant activity to transform GTX2,3 to STX. This activity was stable under heating and protease treatment, showing that it is not an enzymatic reaction. The activity was also observed in the extraction buffer, suggesting that it is the effect of 2-ME contained in the buffer. Figure 8 shows the change of toxin components in the mixture of GTX1,4 and GSH during incubation at 70°C in 0.1 M phosphate buffer (pH 7.4). GTX1,4 decreased markedly and disappeared from the mixture within 2 h. In contrast, neoSTX appeared at 30 min and gradually increased during incubation for 2 h to 35% of the amount of GTX1,4 added to the reaction mixture. GTX1,4 in the phosphate buffer decreased slightly after 12 h, but no neoSTX was detected by HPLC. Similar results were obtained when GTX2,3 was incubated with GSH. In this case, however, STX was formed instead of neoSTX. Figure 9 shows the change of toxin components in the reaction mixture of GTX1,4 and 2-ME during incubation at 70°C in the phosphate buffer (Sakamoto et al. 2000). In this case, most of GTX1,4 disappeared within 2 h while a small amount of neoSTX appeared. The amount of neoSTX increased gradually by 15% of the original amount of GTX1,4. Similar results were obtained from the reaction mixture of GTX2,3 and 2-ME except that STX was formed instead of neoSTX. These results show that thiol compounds transform GTXs to STXs. However, the sum of GTXs and STXs in the reaction mixtures was less than half of the original amount of GTXs, showing that more than half of GTXs added to the reaction mixtures disappeared. This suggests that the conjugates of GTXs and thiol compounds are formed in the course of the transformation. Thus, the 2 h incubation mixtures of GTXs and GSH in which GTXs disappeared, were heated in 1 M 2-ME at 100°C for 10 min. HPLC analysis showed that almost 100% of the added GTXs was recovered as STXs, indicating that GTXs and thiol compounds form conjugates and that additive molecules of thiol compounds are necessary to release STX from the conjugates. doi:10.5047/absm.2010.00301.0001 9 Fig. 8. Change of toxin components in the mixture of gonyautoxins (GTX) 1,4 and glutathione (GSH) during incubation. GTX1,4 (4 µM) was incubated with 1 mL of 8 mM GSH in 0.1 M phosphate buffer, pH 7.4, at 70°C. 䊐, GTX1,4 in the reaction mixture; 䊏, neoSTX in the reaction mixture; 䊊, GTX1,4 in the phosphate buffer as a control. Reprinted with permission from Fisheries Science, 66, Sakamoto et al., Formation of intermediate conjugates in the reductive transformation of gonyautoxins to saxitoxins by thiol compounds. 136– 141, Fig. 3, © 2000, the Japanese Society of Fisheries Science. Fig. 9. Change of toxin components in the mixture of gonyautoxins (GTX) 1,4 and 2-mercaptoethanol (2-ME) during incubation. GTX1,4 (4 µM) was incubated with 1 mL of 8 mM 2-ME in 0.1 M phosphate buffer, pH 7.4, at 70°C. 䊐, GTX1,4 in the reaction mixture; 䊏, neoSTX in the reaction mixture; 䊊, GTX1,4 in the phosphate buffer as a control. Reprinted with permission from Fisheries Science, 66, Sakamoto et al., Formation of intermediate conjugates in the reductive transformation of gonyautoxins to saxitoxins by thiol compounds. 136–141, Fig. 4, © 2000, the Japanese Society of Fisheries Science. Table 2 summarizes the results of TLC of the reaction mixture of GTXs and GSH before and after incubation. After incubation, spots of GTXs disappeared and those of STXs appeared instead. In addition to these, © 2010 TERRAPUB, Tokyo. All rights reserved. 10 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 spots different from those of toxin components and GSH appeared at Rf 0.10 in the mixture of GTX1,4 and GSH and 0.04 in the mixture of GTX2,3 and GSH, respectively, indicating the formation of the conjugates of GTXs and GSH. A spot corresponding to GSH became very faint after incubation, suggesting that excess amounts of GSH were oxidized to GSSG during incubation which could not be detected by this system (Sakamoto et al. 2000). In a trial to purify the conjugate by chromatography on Bio-Gel P-2, it was isolated in a pure form (Sato et al. 2000). The high resolution FABMS and NMR(HMBC) spectrum of the purified substances showed that the compounds are conjugates of neoSTX and thiols in which the C11 atom of neoSTX is covalently bound with a sulfur atom of thiols (Fig. 10). When GTX2,3 was incubated with thiols, the corresponding conjugates of STX and thiols were obtained (Sato et al. 2000a). There has been no report on the reductive elimination of sulfate ester by thiols. Thus, the transformation of GTXs to STXs via the intermediate conjugates is considered to be a quite unique reaction. Formation of the conjugates does not occur under acidic conditions. Furthermore, it was confirmed that GTXs-12-ol reported in toxinproducing cyanobacterium (Onodera et al. 1997) and a dinoflagellate species from tropical area (Lim et al. 2007), analogues in which the gem diol at C12 is reduced, did not react with thiols at all. These facts indicate that the characterstic keto-gem diol structure at C12 of the toxins is essential for the reaction. When the equilibrium mixture of α- and β-O-sulfate toxins is incubated with thiols, the α-O-sulfate toxin is consumed faster than the β-O-sulfate toxin in the formation of the conjugates, showing that α-O-sulfate toxin is a “reactive isomer” (Fig. 11). Treatment of the conjugates with an excess amount of thiols results in the formation of disulfides and STX or neoSTX, indicating that the transformation of GTXs to STXs consists of a two-step reaction (Fig. 11). In the first step, the sulfur atom of the thiols attacks the electrophilic C12 of GTXs to form a thiohemiketal, followed by the formation of stable thioether conjugates by a 1,2 shift. This is an intramolecular reaction in which GTXs easily react with a low concentration of thiols at a low temperature. The second step is an intermolecular reaction between the conjugates and thiols. Therefore, it requires more energy than the first-step reaction. In order to complete the second-step reaction within a short period, heating with an excess amount of thiols is necessary. Actually, the conjugates are formed, even in a solution of GTXs in a low concentration of thiols, such as 1 mM or lower at room temperature. However, only a trace amount of STXs is obtained under this condition. In contrast, almost 100% of STXs in the conjugates can be recovered when conjugates dissolved in 1 M or higher concentrations of thiols are heated for several minutes. Reduction of N1-OH type toxins to N1-H type toxins was not observed in the reaction of GTXs and thiols. This fact does not coincide with the results of Asakawa et al. (1987) in which GSH transformed GTX1 to STX. In their experiments, they used partially purified toxins containing GTX2,3 with a trace amount of GTX1. The reaction mixtures were analyzed by electrophoresis. Probably, most of GTXs in the reaction mixture formed conjugates with GSH and disappeared from the mixture. The amount of neoSTX derived from the conjugate was too low to be detected by electrophoresis. Discovery of stable 11-thiol derivatives of STX is particularly important in terms of the metabolism of PSP toxins. These findings show that STX derivatives are more reactive than previously have been considered. The reaction between GTXs and thiols is useful for the development of biochemical probes such as antibody against PSP toxins which would facilitate the study of more detailed mechanisms of the reactions in which the toxins are involved. Fig. 10. Structure of the intermediates in the transformation of GTX1,4 into neoSTX. Left: gluthathione-neoSTX adduct; right: 2-mercaptoethanol-neoXTX adduct. Reprinted from Bioorganic & Medical Chemistry Letters, 10, Sato, Sakai and Kodama, Identification of thioether intermediates in the reductive transformation of gonyautoxins into saxitoxins by thiols, 1787–1789, © 2000, with permission from Elsevier. doi:10.5047/absm.2010.00301.0001 © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 11 Fig. 11. Mechanism of reductive transformation of GTX1,4 mixture to neoSTX by thiol. Reprinted from Bioorganic & Medical Chemistry Letters, 10, Sato, Sakai and Kodama, Identification of thioether intermediates in the reductive transformation of gonyautoxins into saxitoxins by thiols, 1787–1789, © 2000, with permission from Elsevier. 7. Accumulation and depuration kinetics of PSP toxins in the scallop fed A. tamarense As described above, observation of the field survey on shellfish toxicity in association with the abundance of causative dinoflagellates suggests that the mechanism by which bivalves accumulate PSP toxins is more complex than those which have been considered based on the food web transfer theory. However, it is almost impossible to understand how the shellfish accumulate and depurate PSP toxins during a bloom of dinoflagellates from the field data. Therefore, we examined this problem by the kinetics of toxin accumulation in shellfish based on the feeding experiments of dinoflagellate to the shellfish. In a trial, interspecific difference in the ability to accumulate PSP toxins were examined by rearing five species of bivalves, scallop Patinopecten yessoensis, oyster Crassostrea gigas, mussel Mytilus galloprovincialis, short-necked clam Ruditapes philippinarum, and ascidian Halocynthia roretzi (Sekiguchi et al. 2001a). The specimens of each species were reared in separate tanks with 500 L of seawater and fed a known number of cultured A. tamarense cells. After 24 h, the number of A. tamarense cells in the tank was counted to determine the number of doi:10.5047/absm.2010.00301.0001 ingested cells. At the same time, specimens of each animal were transferred to an aquarium with freshly prepared filtered seawater, to which was added the culture of A. tamarense. The shellfish were then reared in the same way. Specimens of each animal were thus fed on A. tamarense once a day for eight days. After feeding was completed, the specimens were further reared in the same way without feeding for 12 days. During rearing, three specimens of each animal were sampled every two days for the analysis of the toxins by HPLC. All the animals ingested most of the cells fed within 24 h. More than 90% of toxin components of the cells consisted of C1,2 and GTX1,4, and this profile was almost constant throughout the feeding period. Figure 12 shows the change of toxin contents accumulated in each shellfish specimen in a molar base. The amount of each toxin component is also shown in each bar. Average values of accumulated toxins and those of incorporated toxin amounts in a specimen calculated from the toxin contents and ingested cell number of A. tamarense, are also shown in the figure. All the specimens became toxic, but the toxin level of the specimens showed remarkable individual variation, even among the specimens sampled on the same day. To © 2010 TERRAPUB, Tokyo. All rights reserved. 12 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 12. Toxin accumulation in four species of bivalves and an ascidian fed on cultured cells of Alexandrium tamarense. (a) Scallop, (b) mussel, (c) oyster, (d) short-necked clam and (e) ascidian. Reprinted with permission from Fisheries Science, 67, Sekiguchi et al., Accumulation of paralytic shellfish poisoning toxins in bivalves and an ascidian fed on Alexandrium tamarense cells, 301–305, Fig. 1, © 2001, the Japanese Society of Fisheries Science. the contrary, no significant difference was observed in toxin composition of the specimens sampled on the same day. Toxin composition of each animal closely reflected that of A. tamarense, that is, more than 90% of accumulated toxins consisted of C1,2 and GTX1,4. The proportion of C1,2 to GTX1,4 was almost constant during the feeding experiment. These results indicate that there is no difference in the deterioration rate among the toxin components, although C1,2 are reported to be rapidly eliminated over five days in the scallop Patinopecten yessoensis exposed to a natural bloom of A. catenella in which C2 was dominant (Oshima et al. 1990). The difference in the results between feeding experiments and the field study may be due to the difference between natural and laboratory conditions. After the feeding had been stopped, amounts of STXs (STX, neoSTX and decarbamoyl-STX) increased slightly. This trend was observed when the specimens were reared for a longer period. These changes in toxin profile were similar to those observed by Oshima et al. (1990). Although a marked individual difference was observed in the accumulated toxin levels of the three doi:10.5047/absm.2010.00301.0001 specimens sampled at the same stage of the experiment, the average toxin levels showed a trend toward increasing during the feeding experiments. No significant difference was observed in the maximum toxin levels among the mussel, the short-necked clam, and the oyster which were fed on almost equal amounts of A. tamarense (Fig. 12). However, the ascidian accumulated about twice more toxins than other bivalves, although they ingested equal amounts of A. tamarense. Although scallop were fed on three time more than dinoflagellate, they accumulated nearly the same level of toxins as the ascidian. After the feeding had been stopped, toxin levels of each species decreased. However, a considerable high level was often observed in the scallop and the ascidian, even after the cessation of feeding. These results show that accumulated toxins in these animals are hardly eliminated. This observation coincided well with our previous results that scallops maintained a high toxin level for a long time after the disappearance of the causative dinoflagellate in nature. © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Many studies have been carried out to discover the kinetics of toxin accumulation of bivalves (Bricelj et al. 1990, 1991, 1996; Wisessang et al. 1991). In these studies, many shellfish specimens are kept in the same tank and fed on cultured cells of toxic dinoflagellates, and the shellfish toxicity was analyzed by using a homogenate of several specimens to minimize the error of individual differences. The current study unexpectedly revealed that bivalves showed considerable individual variations in toxicity. These differences are considered to be caused by different feeding behaviors among the specimens, probably due to the difference in feeding behavior owing to the sensitivity of handling or a sudden change of environment among the specimens. Thus, the feeding experiments should be designed to take feeding behavior of shellfish into consideration. As handling of the shellfish during the experiments was found to largely affect the feeding behavior of the shellfish (Sekiguchi et al. 2001a), a single specimen of shellfish was reared in a single tank, and the known amount of cultured cells of dinoflagellate was fed to the shellfish specimen (Sekiguchi et al. 2001b). Shellfish accumulate toxins by ingesting the toxic dinoflagellate. Occurrence of toxins in rearing water showed that a part of the toxins was released to the environmental water, even while ingesting the dinoflagellate. When the feeding stopped, the shellfish continuously excreted the toxins. The profile of excreted toxins was similar to that accumulated in the shellfish, indicating that shellfish release the toxin components non-selectively. Interestingly, the amount of toxins accumulated in the shellfish was not parallel to that of the dinoflagellate cells fed to the shellfish (Fig. 13). At the earlier period of the experiment when shellfish were ingesting the cells, the amount of toxins accumulated in the shellfish was more than the supplied toxins from dinoflagellates fed to the shellfish. In the later period when the feeding was stopped, the sum of the toxins in the shellfish and rearing water had decreased to a level which was less than that introduced from the dinoflagellates cells, showing that some of the toxins disappeared from the experimental system. However, this level recovered to almost the same level as that derived from the fed cells of the dinoflagellate, when they were further reared. These facts indicate that a part of the toxins was transformed to an unknown form in the shellfish which could not be detected by chemical analysis such as HPLC applied in our study. The unknown form of the toxins is gradually transformed to toxins again in the shellfish which can be detected by chemical analysis. The unexpected high level of toxins accumulated in the shellfish, more than the fed amount of toxins, may show that an unknown form also occurs in the dinoflagellate fed to the shellfish. Thiol compounds of biological origin, such as GSH, are thought to be involved in the appearance and disappearance of the toxins in shellfish and dinoflagellate. Proteinous thiols doi:10.5047/absm.2010.00301.0001 13 Fig. 13. Change in amounts of PSP toxins accumulated in scallop specimens reared separately in individual tanks. The integrated amounts of toxins released from a single specimen and those supplied to each specimen by feeding Alexandrium tamarense cells are also shown. The sample was accidentally lost during analysis. Reprinted with permission from Marine Ecology Progress Series, 220, Sekiguchi et al., Accumulation and depuration kinetics of paralytic shellfish toxins in the scallop Patinopecten yessoensis fed Alexandrium tamarense, 213– 218, Fig. 3, © 2001, Inter-Research. such as those with a cysteine residue are possibly involved in the formation of the conjugates with the toxins. These thiol-bound toxins possibly react with other thiols in the shellfish and dinoflagellates to release toxins. Chemical transformation of the toxins observed in in vitro experiments could be involved in the transformation in dinoflagellates as well as in shellfish. These findings suggest that PSP toxins undergo a complex metabolism in shellfish as well as in dinoflagellates. 8. Change of toxin productivity in Alexandrium cells There are various data on the toxicity of different strains of A. tamarense isolated from various regions of the world (White and Maranda 1978; Oshima and Yasumoto 1979; Kodama et al. 1982a; Singh et al. 1982; Boyer et al. 1985; Ogata et al. 1987a). As the toxicity of A. tamarense is affected by physiological or environmental factors, these data cannot be directly compared. However, the toxicities of these strains seem to differ from each other. White (1986) reported the high toxin content of A. tamarense in nature. He also demonstrated that the toxicity of the cell in nature fluctuated during the bloom, suggesting that the toxicity of the cells is different in different water masses, even if they bloom in the same area during the same period. Alternatively, these data suggest that the toxicity differs in different clones from one another. Therefore, we examined the toxicity of various clones isolated from the same area during the same season, by culturing them under the same conditions (Ogata © 2010 TERRAPUB, Tokyo. All rights reserved. 14 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 14. Toxicity of various clones of Alexandrium tamarense as a function of growth rate. These clones were grown at 15°C under 3000 lux in medium T1 (Ogata et al. 1987b). Toxicity is expressed in mouse unit (MU) according to Horwitz (1984) where 1 MU represents the dose to kill a 20 g male mouse within 15 min. The growth rate (µ2) is estimated from cell counts during the exponential phase according to the method of Fukazawa et al. (1980). Reprinted from Toxicon, 25, Ogata, Kodama and Ishimaru, Toxin production in the dinoflagellate Protogonyaulax tamarensis, 923–928, © 1987, Pergamon Journal Ltd. with permission from Elsevier. et al. 1987a). Figure 14 shows the toxicity plotted against their growth rate ( µ 2 ) for various clones of A. tamarense which were grown under the same conditions. The data points are randomly scattered, showing that the growth characteristics and toxin production were different, depending on the clone. The maximum differences in toxicity and growth rate were about 100- and 4fold, respectively. HPLC-analysis of the toxin components revealed that these clones had toxin profiles similar to each other. These results indicate the occurrence of various clones of A. tamarense which possess different growth characteristic and toxin productivity. This can be explained by the heterothallism of A. tamarense (Turpin et al. 1978; Anderson 1980; Yoshimatsu 1992). According to this theory, toxin productivity as well as the growth rate of A. tamarense could be inheritance. In order to know the heredity of the toxin productivity of A. tamarense, we established 20 subclones from a single clone isolated from Ofunato Bay. These subclones were grown under the same conditions, as described above, and the toxicity and growth rate were analyzed. In Fig. 15, the toxicities of the subclones are plotted against their growth rate. Most of the subclones showed a similar growth rate. In contrast, their toxicity varied significantly. The maximum difference in toxicity of the subclones was about 20 fold. These results suggest that the toxin production of A. tamarense is not a heredidoi:10.5047/absm.2010.00301.0001 Fig. 15. Toxicity of subclones from a single clone of Alexandrium tamarense (OF84423D-3) as a function of growth rate. These subclones were grown at 15°C under 3000 lux in medium T1. The toxicity (MU) and the growth rate (µ2) are as defined as in Fig. 14. Reprinted from Toxicon, 25, Ogata, Kodama and Ishimaru, Toxin production in the dinoflagellate Protogonyaulax tamarensis, 923–928, © 1987, Pergamon Journal Ltd. with permission from Elsevier. tary character but an acquired one, unless mutation occurred during the culture experiments. In other words, the information on toxin production is not coded in the genes of A. tamarense. These experiments were carried out within one year after the isolation of the clone from which subclones were prepared. Formation of the zygote cannot occur during culture because of the heterothallism of A. tamarense (Turpin et al. 1978; Anderson 1980; Yoshimatsu 1992), indicating that the genetic type of each subclone was identical during our experiments. If mutation occurred, there should have been some groups of clones with different characteristics. However, the toxicities of subclones were different from each other, suggesting that the variation in the toxicity among the subclones is unlikely because of mutation. In conclusion, these results suggest that toxin production of A. tamarense is not a hereditary characteristic, i.e. the information on toxin production is not coded in the genes of A. tamarense. This idea means that toxin production in A. tamarense is an acquired characteristic. Recently, two groups reported separately the same results on nontoxic subclones obtained from a toxic single clone of Alexandrium. Martins et al. (2004) described that possible explanations for the change include genetic mutations or the effects of prolonged treatment of the toxic culture with antibiotics. Omura et al. (2003) describe that they prepared many subclones from non-axenic parent culture of A. tamarense obtained from a single cell © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 15 collected from Ofunato Bay in the same way as described above. Among these subclones, about half were non-toxic. They proposed that this ratio is too large to explain that loss of toxin production in subclones is caused by mutation. Some external factors may be involved in the toxin productivity of toxic dinoflagellates. No significant difference was observed in growth and other physiological characteristics between toxic and non-toxic clones. These facts suggest that PSP toxins are not essential for the survival of these dinoflagellates, but rather are foreign substances for them. 9. Bacterial production of saxitoxin It has been described above that a large difference is observed in the toxicity among various subclones obtained from a single clone. This finding strongly suggests that toxin production of A. tamarense is not a hereditary characteristic. Bacterial symbiosis or infection is commonly observed in higher plants. Thus, it is plausible that the bacteria are involved in toxin production of A. tamarense. A strongly toxic clone of A. tamarense was treated with a mixture of antibiotics to obtain the axenic culture. The culture was tested for the presence of bacteria and confirmed that the culture medium was bacterium-free. A. tamarense cells were harvested from thus-obtained bacteria-free culture, washed several times with sterilized seawater, lightly homogenized, and then inoculated onto agar plates of a nutrient medium. After incubation for several days, bacterial colonies were observed on the plates. The obtained bacterial strain was mass-cultured and bacterial cells were extracted for toxin analysis. As the toxicity was detected by mouse bioassay, the extract containing crude toxin was prepared from more than 70 L of culture. The toxic substance was isolated by using several types of chromatographies. The purified substance was indistinguishable from authentic STX in the analysis by different types of chromatographies such as TLC, electrophoresis, and HPLC for PSP toxins (Kodama et al. 1988). Typical results from HPLC analysis are shown in Fig. 16. The peak height in HPLC to the mouse toxicity ratio also coincided well with that of standard STX, showing that the obtained bacterium produces STX. This toxigenic bacterium (PTB-1) was tentatively identified as “Moraxella sp.” [later classified as a Pseudomonas/ Alteromonas species (Doucette and Trick 1995)] and most recently as a new genus within the α-proteobacteria (Kopp et al. 1997). Doucette and Trick (1995) and Franca et al. (1995) employed HPLC methods to examine toxin production characteristics of Moraxella sp. and another bacterium obtained from the toxic dinoflagellate A. lusitanicum. Both groups reported PSP toxins in bacterial cell extracts. Gallacher and co-workers have made an important step in confirming the synthesis of PSP toxins by bacteria isolated from five Alexandrium cultures, doi:10.5047/absm.2010.00301.0001 Fig. 16. Identification of the bacterial toxin as saxitoxin by HPLC-fluorometric analysis. A, saxitoxin standard (0.2 MU); B, bacterial toxin (0.3 MU). Reprinted from Agricultural and Biological Chemistry, 52, Kodama et al., Bacterial production of saxitoxin, 1075–1077, © 1988, the Agricultural Chemical Society. including the A. lusitanicum strain noted above (Gallacher et al. 1997), using the capillary electrophoresis-mass spectrometry (CE-MS) method. On the other hand, Silva (1979) presented the hypothesis that dinoflagellate toxicity is due to intracellular bacteria. We also suggested that toxin production by A. tamarense is not of hereditary characteristics (Ogata et al. 1987b). The finding of toxin-producing bacterium in the cells of A. tamarense support both Silva’s hypothesis and our previous results. The toxin production by our bacterium was considerably lower than that by A. tamarense, suggesting that symbiosis or parasitism with A. tamarense enhances the toxin production by this bacterium. In addition, only STX could be detected in Moraxella sp., whereas the main toxin components of A. tamarense are GTXs (Ogata et al. 1987a, b). Thus, the toxin production of Moraxella sp. under various condition was surveyed. As shown in Fig. 17, Moraxella sp. in shaking culture grew faster than those in standing or rotatory culture at 25 and 37°C. Figure 18 shows time course of toxicity in the cell extract of Moraxella sp. grown under various culture conditions. No significant toxicity was observed in any culture during 64 h. At 25°C, it increased during 24 h, whereas it decreased after 24 h. The cells shaken in Tryptic-Soy Broth (TSB; BBL Co. Ltd. Tokyo) at 25°C also showed weak toxicity at 64 h which disappeared after 168 h incubation. On the other hand, Moraxella sp. showed considerable toxicity in all the culture conditions when cultured in Marine Broth (MB; Difco) at 25°C for 168 h. Inoculates of Moraxella sp. in 1% MB and seawater became slightly turbid after 10 days incubation, showing that the bacterium grew only slightly in these media. Figure 19 illustrates HPLC chromatograms of the extract of Moraxella sp. grown in the seawater for 10 days. Peaks © 2010 TERRAPUB, Tokyo. All rights reserved. 16 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 17. Growth of Moraxella sp. Under various culture conditions. Moraxella sp. Was cultured in Heart Infusion (䊊), Brain Heart Infusion (䊉), Tryptic-Soy Broth (䉭), CAYE medium containing 1% Casamic acid and 1% yeast extracts (䉱), and Marine Broth (䊏) at 25 and 37°C by standing, shaking and rotatory culture methods. Upper, 25°C, Lower 37°C; A, standing culture; B, shaking culture; C, rotatory culture. Reprinted from Toxicon 28, Kodama et al., Production of paralytic shellfish toxins by a bacterium Moraxella sp. isolated from Protogonyaulax tamarensis. 707–714, © 1990, Pergamon Press plc. with permission from Elsevier. Fig. 18. Time course of toxin production by Moraxella sp. under various conditions. The toxicity of cells grown under various conditions (refer to Fig. 17) was assayed by mouse neuroblast cell culture method (Kogure et al. 1988; Sato et al. 1988). Upper, 25°C; Lower, 37°C; A, standing culture; B, shaking culture; C, rotatory culture. Reprinted from Toxicon 28, Kodama et al., Production of paralytic shellfish toxins by a bacterium Moraxella sp. isolated from Protogonyaulax tamarensis. 707–714, © 1990, Pergamon Press plc. with permission from Elsevier. doi:10.5047/absm.2010.00301.0001 © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 19. HPLC-fluorometric analysis of the extract from Moraxella sp. cells cultured in natural seawater for 10 days at 20°C, using a develosil C8 column according to the method of Oshima et al. (1987). Mobile phase in (A) and (C) is 2 mM 1-heptanesulfonic acid in 10 mM phosphoric acid (pH 7.2). Mobile phase in (B) and (D) is 2 mM 1-heptanesulfonic acid in 10 mM phosphoric acid containing 10% acetonitrile (pH 7.2). (A), GTX standards: GTX1 (5 pmoles); GTX2 (1.3 pmoles); GTX3, (0.5 pmoles); GTX4, (12 pmoles). (B): neoSTX and STX standards; neoSTX, (9 pmoles); STX, (5 pmoles). (C) and (D): extract of Moraxella sp. (10 µL of extract corresponding to cells in 5 mL of culture). Reprinted from Toxicon 28, Kodama et al., Production of paralytic shellfish toxins by a bacterium Moraxella sp. isolated from Protogonyaulax tamarensis. 707– 714, © 1990, Pergamon Press plc. with permission from Elsevier. corresponding to GTX1,4 followed by those of GTX2,3 appeared. A faint peak corresponding to neoSTX was also observed. These results show that main toxin components of Moraxella sp. grown under these conditions are similar to the GTXs. Figure 20 and Table 3 show the changes of total toxin amounts per 1 L of culture and proportion of toxin components which were calculated from the results of HPLC-fluorometric analysis. In the seawater, Moraxella sp. produced PSP toxins within 24 h, however, the amount was low for one week, but increased considerably by the 10th day. The pattern of toxicity change in 1% MB was similar to that in the seawater, although the toxicity did not increase as in the seawater. Throughout the incubation period the toxin profile changed (Table 3). However, the main components were always similar to GTXs, especially GTX1,4. In the extreme cases, most of the toxin was GTX1,4. STX and neoSTX were scarcely observed. These findings suggest that transformation of toxin components occurs in Moraxella sp. doi:10.5047/absm.2010.00301.0001 17 Fig. 20. Changes of total toxin amount during culture. 䊉: culture in 1% Marine Broth; 䊊: culture in natural seawater. Reprinted from Toxicon 28, Kodama et al., Production of paralytic shellfish toxins by a bacterium Moraxella sp. isolated from Protogonyaulax tamarensis. 707–714, © 1990, Pergamon Press plc. with permission from Elsevier. These results show that Moraxella sp. required sodium chloride for its growth and grew best in shaken cultures, indicating that it is an aerobic marine heterotrophic bacterium. This fact is also supported by stable growth under various conditions in MB which is prepared from artificial seawater. However, during 64 h, it did not produce significant amount of toxin under these culture conditions. Only cells of the standing culture in Heart Infusion (HI) at 25°C showed significant toxicity although the culture grew poorly under this condition. Interestingly, once produced, toxin often disappeared during incubation. Although it is poorly understood about the metabolism of PSP toxins, these findings suggest that the toxin is an intermediate which is metabolized to non-toxic substance(s). Chemical reactions between GTXs and biological thiols such as GSH are considered to be involved in these phenomena. On the other hand, the toxin appeared in MB cultures which were grown for 168 h at 25°C. The cells at 168 h incubation should be under a starved condition, because they were left under a nutrition deficient environment for more than 100 h. In order to obtain energy for survival under starved conditions, Moraxella sp. possibly catabolizes its biological substance(s). PSP toxins might be a kind of catabolites of the metabolism (Kodama et al. 1990b). 10. Bacteria in the cells of toxic dinoflagellates Several studies indicate that axenic, or bacteria-free, dinoflagellate cultures retain the ability to produce these © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 18 Table 3. Toxin composition of Moraxella sp. cultured seawater and 1% marine broth. Toxin composition* Duration GTX1 Culture medium (days) mole % GTX2 GTX3 Natural seawater 1 2 3 4 5 6 7 10 41.0 25.6 100.0 50.5 100.0 70.5 56.7 10.0 11.8 8.0 — 8.7 — 23.7 — 0.5 2.7 2.2 — 1.8 — 5.8 — 1.4 1% Marine Broth 1 2 3 4 5 6 7 10 28.1 20.3 37.0 1.0 — 34.8 90.7 40.7 — 6.2 — 0.1 — 4.4 9.3 10.4 2.5 1.8 — 3.4 — — — 1.6 GTX4 neoSTX STX 44.5 64.2 — 28.7 — — 43.3 87.3 — — — — — — — 0.7 — — — — — — — — 69.4 71.7 63.0 95.5 100.0 60.8 — 47.3 — — — — — — — — — — — — — — — — *Toxin composition was calculated by comparing peak heights of samples against standard PSP toxins and expressed as mole %. This article was published in Toxicon, 28, Kodama, Ogata, Sakamoto, Sato, Honda and Miwatani, Production of paralytic shellfish toxins by a bacterium Moraxella sp. isolated from Protogonyaulax tamarensis, 707–714, © Elsevier (1990). toxins at a level similar to those found in nonaxenic cultures (e.g. Kim et al. 1993). If bacteria are involved in toxin production in dinoflagellates, they should be present within the dinoflagellates. Several investigators including us have reported the direct observation of intracellular bacteria in toxic species of dinoflagellates by electron microscopy (Silva 1979; Kodama and Ogata 1988; Franca et al. 1996), although the bacterial numbers were generally few and such bacteria were not always discernible even with cells of the same algal culture. Although toxigenic bacteria could be isolated from toxic dinoflagellates, it was not clearly proven whether the isolated bacterial strains and the corresponding intracellular bacteria were the same. In order to know the presence of intracellular bacteria in the toxic A. tamarense, we used an antibody against a toxin-producing bacterium to perform Western blots of an extract from the “host,” an A. tamarense isolate (axenic culture) (Kodama et al. 1996a). Increased cross reactivity was observed after the apparent disruption of residual (=accumulation) bodies present within the algal cells. Figure 21 shows the results from western blot analysis using antibody against Moraxella sp. and the extract of A. tamarense cells which was extracted mildly with phosphate buffered saline (PBS) using a Teflon homogenizer. Several faint bands were observed, doi:10.5047/absm.2010.00301.0001 Fig. 21. Western blot analysis of the extract of Alexandrium tamarense prepared by a Teflon homogenizer using antiMoraxella sp. antibody. A: extract of Moraxella sp., B: extract of A. tamarense. Reprinted from Kodama et al., Symbiosis of bacteria in Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 351–354, Fig. 1. some of which corresponded with those of Moraxella sp. extract, suggesting the presence of protein components of Moraxella sp. in A. tamarense cells. However, the number of the bands was too small to show clearly the presence of bacterial protein components in A. tamarense cells. As the protein components were considered to be not sufficiently extracted, the homogenate to prepare the extract was observed under light microscope. As shown © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 22. Microscopic observation of the homogenate prepared by a Teflon homogenizer. Upper: light microscopic observation; lower: fluorescent microscopic observation. Reprinted from Kodama et al., Symbiosis of bacteria in Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 351–354, Fig. 2. 19 in Fig. 22, most of the cells were disrupted, but many intact organelle were observed. These organelle showed fluorescence when activated by ultraviolet light, showing that the organelle were hard to be homogenized by the method applied. Therefore, homogenate of A. tamarense was sonicated for 1, 5 and 10 min in phosphate buffered saline (PBS) in the same buffer containing 2% SDS. Figure 23 shows the observation of the homogenates under fluorescent microscope. Although most of the cells were disrupted by 1 min sonication, many fluorescent organelle were not disrupted by the procedure in both buffers. Significant number of these organelle were still observed even after 10 min sonication. However, the number decreased considerably, showing that they were disrupted as the sonication time became longer (Fig. 23). SDS was effective for the disruption of the organelle. Western blot analysis of the extracts prepared from these homogenates showed that the staining of the bands with antibody against Moraxella sp. became stronger as the sonication time of the cells in SDScontaining buffer for longer time was effective to extract the fluorescent organelle. Therefore, a large number of the cells (5 × 107 cells) were extracted with 0.5 mL of 1.25 M tris containing 10% SDS to prepare the concentrated extract. The extract thus obtained showed clear bands corresponding to almost all the protein components of Moraxella sp. in western blot analysis (Fig. 24). These results show that protein components which reacted with antibody against protein extracts of Moraxella sp. are contained in these fluorescent organelle. A. tamarense Fig. 23. Fluorescent microscopic observation of homogenates prepared under different conditions. Upper: Cells were sonicated for 1, 5, 10 min in SDS-containing buffer; lower: Cells were sonicated for 1, 5, 10 min in PBS. Bar: 100 µm. Reprinted from Kodama et al., Symbiosis of bacteria in Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 351–354, Fig. 3. doi:10.5047/absm.2010.00301.0001 © 2010 TERRAPUB, Tokyo. All rights reserved. 20 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 24. Western blot analysis of the extract of Alexandrium tamarense prepared by sonication in SDS-containing buffer. A: extract of Moraxella sp.; B: extract of A. tamarense. Reprinted from Kodama et al., Symbiosis of bacteria in Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 351– 354, Fig. 4. clone used in the present study was grown and maintained for more than a year under axenic condition. No bacteria were observed in the cell-free medium of the clone in monthly test for detection of bacteria. Therefore, the presence of bacterial substances in the extract of A. tamarense clone implies that bacteria are living in the cells, probably in the fluorescent organelle. Figure 25 shows the light and fluorescent microscopy of A. tamarense cell harvested at late stationary phase. Autofluorescent organelle is seen under the nucleus. The organelle showed blue-white fluorescence under UV light excitation, green fluorescence under blue light excitation with a red cut-off filter and yellow without the red cut-off. These characteristics are similar to those of autofluorescent organelle called PAS/accumulation bodies observed in other dinoflagellates which are considered to be lysosome. Franca et al. (1995) and Mascarenhas et al. (1995) have suggested that residual bodies of A. lusitanicum which seem to be the same as PAS/accumulation bodies, contain remnant of bacteria. They observed living bacteria in the space between the outer cellular membrane and the thecal complex, which seem to originate from environment. From these facts, they suggested that incorporated bacteria are trapped by food vacuole and transferred to the residual body to be digested. The digested bacteria might be a nutritive source for Alexandrium. On the contrary, the results of the present study show that bacteria are living in the Alexandrium residual body. Therefore, a part of the bacteria incorporated into the inside the cells seem to survive and grow in the organelle while others are digested in the organelle. It is well known that organic substances such as soil extracts and vitamins are essential for the growth of Alexandrium. We suggest that A. tamarense can grow by utilizing bacteria as well as organic substances under limitation of nutrient or light intensity (Ogata et al. 1996). These findings suggest that Alexandrium spp. require organic substances which originate from bacteria. Under doi:10.5047/absm.2010.00301.0001 Fig. 25. Microscopic observation of Alexandrium tamarense cell. Left: light microscopic observation; right: fluorescent microscopic observation. Reprinted from Kodama et al., Symbiosis of bacteria in Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 351–354, Fig. 5. axenic condition in which no bacteria are supplied from the environment, a part of bacteria growing in the lysosome-like organelle are considered to be digested in the organelle to provide an organic substance to A. tamarense for its growth. The relation between A. tamarense and endocellular bacteria may be mutualism. These results and the facts that bacteria could be isolated from an axenic culture of this dinoflagellate only after disruption of the residual bodies, led us to contend that intracellular bacteria exist within the residual bodies and these retain the ability to reproduce within the algal cells. This idea is consistent with electron microscopic observations showing partially digested bacteria in the residual bodies of several toxic dinoflagellates (Franca et al. 1995). The fact that apparently stable association of certain bacteria with an algal isolate is observed over many years also support the idea. Generally, bacteria responsible for infection are killed by defense mechanisms of the host organism. However, some specific infectious bacteria are known to possess escape mechanisms allowing them to survive within cells of the host (Groisman 1992). It is hypothesized that the growth of such bacteria inside residual bodies may be controlled by digestive processes, thereby harmonizing bacterial growth with that of the dinoflagellate and allowing extended maintenance of the intracellular-bacterial flora. 11. Utilization of organic substances for growth and toxin production by Alexandrium tamarense There have been many dinoflagellate species reported to possess a heterotrophic behavior such as © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 26. Growth curve of Alexandrium tamarense cultured semicontinuously in N-limited T1 medium in which nitrate level was reduced to 2% of the normal medium. A half volume of culture was diluted with equal volume of fresh medium successively when the cell density increased to double. Reprinted from Ogata et al., Utilization of organic substances for growth and toxin production by Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 343–346, Fig. 1. auxotrophy or phagotrophy (Gaines and Elbrachter 1987). However, dinoflagellates which produce PSP toxins such as A. tamarense have until now been regarded as photosynthetic organisms. Therefore, their growth physiology and toxin production have been studied from the standpoint that they are autotrophic organisms. As described above, we gave a clear evidence that photosynthesis is necessary for the toxin production of A. tamarense (Ogata et al. 1987b). The fact also supports the autotrophic character of A. tamarense. On the other hand, Alexandrium spp. require some organic substances such as vitamins (Iwasaki 1979), indicating that they also possess heterotrophic characters. However, little is known about this process on these species. We found that A. tamarense possesses bacteria in the cells and that the isolated bacteria produce toxins, though the productivity is low (Kodama et al. 1988). Based on these results, we propose a hypothesis that these bacteria are necessary for A. tamarense to produce toxins. Thus it is based on a premise that A. tamarense utilizes organic substance. Such heterotrophic character is considered to be also important to understand the growth of these species in the natural environment. Despite numerous reports on the relationship between the occurrence of Alexandrium spp. and environmental factors such as inorganic nutrient concentrations, it seems difficult to correlate the growth of this dinoflagellate solely to these factors. The utilization of organic substance(s) of A. tamarense was studied in relation with growth and toxin production of this species (Ogata et al. 1996). The unialgal and axenic cultures were maintained under irradiation of 60 µmol photons·m –2·s–1 (cool-white fluorescent lamps) with 16 h:8 h LD cycle. doi:10.5047/absm.2010.00301.0001 21 T1 and SWII medium (nitrate; 0.71 mM) (Iwasaki 1961) were used for unialgal and axenic cultures, respectively. Semi-continuous culture was established by repeating dilution of culture with an equal volume of fresh medium when the cell density increased to the double. An aliquot of the cells was taken to measure cell density under a microscope. The growth rate (µ 2) was estimated by the method of Fukazawa et al. (1980). Toxin contents of the cultured cells were analyzed by HPLC according to Oshima (1995b). As shown in Fig. 26, the semi-continuous culture acclimated to N-limited condition was established by cultivating A. tamarense in N-limited T1 medium in which nitrate was reduced to 2% of the normal medium. During the first 30 days, the growth of A. tamarense was unstable, but it showed stable growth with growth rate of 0.25 division/day after 30 days. The culture maintained exponential phase, showing that cells grew continuously under N-limited condition. As shown in Fig. 27, toxin content of the cell dropped to about 1/10 that of those in the initial culture cultivated in normal T1 medium as indicated previously (Boyer et al. 1987; Anderson et al. 1980). When nitrate (1.0 mM) or yeast extract (DIFCO; 20 mg·L–1) was added to the culture (indicated by arrows in Fig. 27), the cells grew well. The maximum yield of the culture increased remarkably in both cases. At the same time, toxin production of the cells was enhanced to almost 5 times by these treatments. Nitrate and ammonia in yeast extract used here were measured to be 0.055 and 1.095 µmol·g–1, respectively, being too low to explain the growth after the addition of yeast extract. These results show that A. tamarense can use organic N-substance in yeast extract as the nitrogen source instead of nitrate. Figure 28 also shows the effect of bacterium, Moraxella sp., on the growth and toxin production of A. tamarense cultured under N-limited condition semicontinuously. At the dilution of the culture in the maximum density, 107 cells of live or autoclaved Moraxella sp. cells were added with 100 mL of the fresh medium. The maximum cell number of A. tamarense increased significantly by addition of autoclaved bacterial cells, as in the case of yeast extract. This increase in growth was repeatedly observed. Toxin production of cells was also induced by this treatment. In contrast, growth of A. tamarense became lower by the addition of live Moraxella sp. cells. However, when the addition of bacterial cells was repeated, A. tamarense started to grow well as in the case of autocraved cells (0.39 division/day). Toxicity of cells also increased gradually by the treatment. These results indicate that A. tamarense also possess phagotrophic characters and utilize environmental bacteria for their growth and toxin production. As mentioned above, Franca et al. (1995) observed bacteria in the space between the outer cellular membrane and the thecal complex, and suggested that these bacteria might © 2010 TERRAPUB, Tokyo. All rights reserved. 22 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 27. Change of growth (lower) and toxin production (upper) of Alexandrium tamarense after addition of nitrate (1.0 mM) or yeast extract (20 mg·L–1) under N-limited condition. Nitrate and yeast extract were added at a time indicated by arrow. N: Culture supplemented with nitrate, Y: Culture supplemented with yeast extract, B: Control culture. Reprinted from Ogata et al., Utilization of organic substances for growth and toxin production by Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 343–346, Fig. 2. Fig. 28. Change of growth (lower) and toxin production (upper) of Alexandrium tamarense after addition of autoclaved (AM) or live (LM) Moraxella sp. cells under N-limited condition. Bacterial cells were added at times indicated by arrows. Reprinted from Ogata et al., Utilization of organic substances for growth and toxin production by Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 343–346, Fig. 3. doi:10.5047/absm.2010.00301.0001 © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 23 be a nutritive source of the dinoflagellate. Our results support their observations. Previously, we have reported that de novo synthesis of toxins in A. tamarense is repressed under low light condition (Ogata et al. 1987b). Therefore, the effect of organic substance on the toxin production under low light condition was examined. As shown in Fig. 29, cell division of A. tamarense stopped at midexponential phase by lowering light intensity from 60 to 5 µmol photons·m–2·s–1. No significant toxin production was observed during 4 days under this condition. When the yeast extract (20 mg·L–1) was added to the culture at the time of lowering light intensity, cells were observed to keep growing though the growth rate became lower. During the period, the toxin contents of cell increased. These results suggest that A. tamarense can grow by utilizing organic substances such as yeast extract, if they are available, even when it can not grow autotrophically under low light condition. Cultures used in these experiments were not axenic. It could be possible that bacteria contaminating the culture decomposed the added organic substances to inorganic nutrients which are available for A. tamarense. Therefore, the effect of organic substance on the growth was examined by using an axenic culture. As shown in Fig. 30, the axenic strain grew well in SWII medium with the maximum yield of 8 × 104 cells·mL–1. Cells grown in N-depleted SWII medium (SWII-N) showed a similar growth, but the maximum yield was much depressed to 2 × 10 3 cells·mL–1. When the culture was spiked with nitrate (1.0 mM) at the stationary phase, N-limited culture induced remarkable cell division, resulting in the recovery of cell yield of 8 × 104 cells·mL–1. Growth of the cells in SWII-N was also induced by the addition of yeast extract (20 mg·L–1), though the growth rate and cell yield were lower than those of nitrate addition. These results clearly show that A. tamarense is mixotrophic organism. It can grow autotrophically under the conditions suitable for photosynthesis. When the environment is not suitable for photosynthesis, it can grow using energy Fig. 29. Changes of growth (lower) and toxin production (upper) of Alexandrium tamarense by addition of yeast extract (20 mg·L–1) under light limited condition. Culture was divided to two parts after lowering light intensity at mid-exponential phase. A: Control culture, B: culture supplemented with yeast extract at the time indicated by arrow. Reprinted from Ogata et al., Utilization of organic substances for growth and toxin production by Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 343–346, Fig. 4. Fig. 30. Effect of supplemented yeast extract on the growth of axenic Alexandrium tamarense cells. Cells were grown in SWII medium (䊐), N-depleted SWII (䉱), SWII supplemented with nitrate (1.0 mM) (䉬), and SWII supplemented with yeast extract (20 mg·L–1) (䊊). Nitrate and yeast extract were added at the time indicated by arrow. Reprinted from Ogata et al., Utilization of organic substances for growth and toxin production by Alexandrium tamarense. In: Yasumoto, Oshima and Fukuyo (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996, pp. 343– 346, Fig. 5. doi:10.5047/absm.2010.00301.0001 © 2010 TERRAPUB, Tokyo. All rights reserved. 24 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 heterotrophically supplied. It is striking that A. tamarense produces toxins even when it grows heterotrophically. This fact is very important to understand the blooming mechanism of Alexandrium species. Although a number of survey have been conducted on the environmental factors for the growth of natural population of A. tamarense, little is known about their blooming mechanisms. In the field survey in Ofunato Bay, the bloom of A. tamarense was often observed under the environment in which the level of inorganic N such as nitrate or ammonia was low (unpublished data). During the bloom, this species was found in higher densities at around 10 m depth where light intensity was as low as 30 µmol photons·m–2·s–1 even during sunny day. These facts indicate that natural population of A. tamarense grows in an environment in which its autotrophic activity is limited. Jacobsen and Anderson (1996) presented electron micrographs clearly showing that A. ostenfeldii ingests the plankton as food. These findings suggest that heterotrophic characters as well as autotrophic ones play an important role for the growth and toxin production of the natural population of Alexandrium species. 12. Toxin-producing bacteria in marine ecosystem Since the first finding that a bacterium (PTB-1) isolated from A. tamarense produces STX (Kodama et al. 1988; Doucette and Trick 1995), several strains of STXproducing bacteria have been isolated from dinoflagellates (Franca et al. 1996; Gallacher et al. 1997). Also we have detected STXs in fractions of particles smaller in size than dinoflagellates (Sakamoto et al. 1993). In a monitoring survey on shellfish toxicity, we analyzed STXs in particles suspended in seawater after fractionation of the particles according to size by passing them through sieves of different pore sizes. Figure 31 shows data obtained for samples from Tanabe Bay where shellfish became toxic during a bloom of A. catenella (Sakamoto et al. 1992, 1993). Surprisingly, no significant amount of shellfish toxicity increased considerably (indicated by arrows in Fig. 31). In contrast, significant toxicity was detected in association with particles smaller than A. catenella such as those in the size ranges of 5–20 and 0.45–5 µm by HPLC analysis. The 5–20 µm fraction showed the highest level of toxicity when the cell density of A. catenella was very low. The toxins in this fraction were highly purified and identified as STX by mass spectrometry. These findings clearly indicate that an unknown STX-producing organism(s), smaller than A. catenella, occurs together with A. catenella in the seawater. Similar results were obtained for samples from Ofunato Bay during a bloom of A. tamarense (Kodama et al. 1990a). A slight increase in shellfish toxicity was observed when fractions containing particles smaller than dinoflagellates showed considerable toxicity. As no significant toxicity was observed in other particle fractions, doi:10.5047/absm.2010.00301.0001 the shellfish toxicity during the period seemed to be due to the toxins associated with these particles. However, shellfish toxicity did not increase remarkably, although high levels of STXs were detected in the fractions containing particles with a size smaller than dinoflagellates. It seems quite strange that shellfish toxicity increased significantly as A. catenella grew to high density; yet no significant amount of toxin was detected in A. catenella (Sakamoto et al. 1993). Although definitive proof of a bacterial source of the toxin is lacking, STXs detected in particles smaller in size than dinoflagellates are thought to originate from bacteria grown in seawater, and thus the particles may be living on the surface of detritus which is favorable for their growth (Kodama et al. 1990a). Probably, as the large number of bacterial cells are attaching on the detritus, the amount of toxin in the detritus is often greater than the amount associated with free-living bacteria. However, even though the detritus fraction (5–20 µm fraction) showed high toxicity, shellfish toxicity did not increase remarkable. These findings may indicate that bacterial toxins are not directly associated with shellfish toxicity. It is also noteworthy that, although shellfish became toxic during a bloom of A. catenella, no significant toxicity was detected in the A. catenella cells from this location (See Fig. 31). These findings support the idea that the toxicity of shellfish cannot be attributed solely to transport of dinoflagellates toxins via the food chain. Toxinproducing bacteria may possess “biological component” in which toxin is incorporated as a part; the “biological component” which releases toxin by digestion. The distribution of STX-producing bacteria in the marine environment appears to be quite widespread. Investigators in increasing numbers are now reporting the isolation of such bacteria from locations that are spatially and temporally distinct from the locations of the dinoflagellates. Levasseur et al. (1996) examined the potential for bacterial production of STXs in the St. Lawrence estuary, Canada. They screened bacterial strains isolated from the St. Lawrence estuary by HPLC and found four strains positive for STXs, indicating that putatively toxigenic bacteria are present in that region. Interestingly, these four bacterial strains isolated from the St. Lawrence estuary by Levasseur et al. (1996) originated from sites exhibiting no concurrent PSP activity, and three of these locations had no prior history of bloom formation. Although free-living and particle associated bacteria capable of producing STXs appear to exist in the St. Lawrence estuary, only the toxins associated with larger size fractions accounted for shellfish toxicity during this Alexandrium bloom. These results indicate that STX-producing bacteria ingested by shellfish could not be directly responsible for the STXs in the shellfish, although a contribution of toxin-producing bacteria to shellfish toxicity could not be ruled out. © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 31. Seasonal variation of Alexandrium catenella abundance, scallop toxicity and the toxicity of particle fractions from January to June 1990 in Tanabe Bay. (A) Vertical distribution of A. catenella. Cell density (cells L–1): (䊐) 0 to 99; (䊐) 100 to 999; (䊐) 1000 to 4999; (䊐) 5000 to 9999; (䊐) >10000. (B) Abundance of A. catenella (䊉) (no of cells) and scallop toxicity (䊊, MU g–1). A. catenella were collected from 1 L samples taken every 2 m from the surface to 8 m depth. Arrows: peaks of A. catenella abundance where shellfish toxicity is decreasing. (C) Toxicity of particle fractions: (䊐) > 20 µm; (䉱) 5–20 µm; () 0.45–5 µm. Reprinted with permission from Marine Ecology Progress Series, 89, Sakamoto et al., Causative organism of paralytic shellfish toxins other than toxic dinoflagellates, 229–235, Fig. 1, © 1992, Inter-Research. doi:10.5047/absm.2010.00301.0001 © 2010 TERRAPUB, Tokyo. All rights reserved. 25 26 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Information on the distribution of STX-producing bacteria in marine ecosystems is important to solve the problem of the association between these bacteria and the STXs of shellfish and other organisms. In order to prove the toxigenicity of bacteria, more detailed chemical evidence such as analytical data obtained by mass spectrometry or nuclear magnetic resonance (NMR) are required. However, because of the low toxin productivity of bacteria, various investigators have applied indirect methods for toxin identification such as HPLC and bioassay of crude extracts. When crude extracts are analyzed by fluorometric-HPLC, ghost peaks with retention times identical to those of standard toxins often appear (Onodera et al. 1997). However, these peaks can be distinguished from toxin peaks by changing the reaction reagent for post-column derivatization to water; the ghost peaks do not disappear as a result of this treatment, but the toxin peaks disappear. In addition, these impurities can be mostly removed by pretreatment of the samples, such as by partial purification of the toxins by Bio-Gel P2 (Bio-Rad Laboratories, Richmond, CA) or activated charcoal treatment. HPLC analysis of bacterial toxins after such treatment will provide more detailed information concerning the toxigenicity of the bacteria. 13. STXs in organisms other than dinoflagellates and their associated organisms It is well known that STXs are found not only in dinoflagellates and their associated shellfish but also in phylogenically diverse species of animals. Noguchi et al. (1969) showed for the first time that the xanthid crab Atergatis floridus, a non-plankton feeder animal accumulates a high level of STX which could not be associated with dinoflagellates. After this discovery, high levels of STXs were detected in various animals, such as the horseshoe crab, Carcinoscorpius rotundicauda (Fusetani et al. 1982) and several species of marine snails (Kotaki et al. 1981). We also found that a low level of STX is detected in puffer Takifugu pardalis in addition to a large amount of tetrodotoxin (TTX) (Kodama et al. 1983a). In 1988, a food poisoning including fatal cases by ingestion of freshwater puffer Tetraodon fangi occurred (Laobhripart et al. 1990). We analyzed the puffer by mouse assay and found that the puffer possesses a potent neurotoxin, though no TTX was detected by chemical analysis (Sato et al. 1997). In the analysis of PSP toxins by HPLC, however, a large amount of a toxin was detected instead of TTX which was identified as STX (Sato et al. 1997) (Fig. 32). Although no intensive survey on the PSP toxins-bearing organisms was conducted, toxins were not detected in freshwater bivalves collected from the area where the puffer were observed. Thus, the source of STX found in freshwater puffer is unknown. In order to know the occurrence of STXs in puffer, we surveyed toxins in tropical species of puffer on which there is few knowledge (Sato et al. 2000a). Seven species of puffer including 27 specimens of Arothron Fig. 32. Fast bombardment mass spectrum of freshwater puffer toxin. A fast atom bombardment mass spectrum (FAB-MS) of the purified toxin was measured in glycerol as a matrix through a magnetic scan from 25 to 500 mass units. The toxin exhibited an ion peak at m/z = 300, 282 and 374, which correspond with a pseudomolecular ion (M + H)+ of authentic STX, (M + H – H2O)+ and (M + H + glycerol – H2O)+, respectively. Reprinted from Toxicon, 35, Sato, Kodama, Ogata, Saitanu, Furuya, Hirayama and Kakinuma, Saxitoxin as a toxic principle of a freshwater puffer Tetraodon fangi, in Thailand, 137–140 © 1997, Pergamon Press plc. with permission from Elsevier. doi:10.5047/absm.2010.00301.0001 © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 27 Fig. 33. Toxicity of various organs of puffers collected from Masinloc Bay, Philippines. The toxicity was measured by mouse bioassay and expressed as mouse unit (MU) of TTX. L: liver, I: intestine, M: mussel, S: skin. (a) Number of specimens which did not kill mice (ND): 2, (b) 2, (c) 1, (d) 3, (e) 1, (f) 2, (g) 1, (h) 5, (i) 1. Reprinted from Toxicon, 38, Sato, Ogata, Borja, Gonzales, Fukuyo and Kodama. Frequent occurrence of paralytic shellfish poisoning toxins as dominant toxins in marine puffer from tropical water, 1101–1109, © 2000, Pergamon Press plc, with permission from Elsevier. mappa, 25 of A. manillensis, 20 of Chelonodon patoca, 6 of A. nigropunctatus, 5 of A. hispidus, one A. stellatus and one A. reticularis were collected at Masinloc Bay, Luzon, Philippines over the year of 1992. The tissues excised from partially thawed specimens were subjected to extraction and analyzed by HPLC for PSP toxins as well as TTX. Figure 33 shows the toxicity of the extracts analyzed by mouse bioassay. Most of the extracts killed mice with typical signs of TTX and STXs. In the chemical analysis, a considerable level of STXs was detected in addition to TTX. Although the number of the specimens was not enough, the major toxin of these puffer seemed to be STXs. In Fig. 34 is shown one of the results obtained from A. mappa specimen by HPLC analysis. In addition to TTX, significant level of neoSTX, decarbamoyl STX, GTX5 and STX were detected. Most of the puffer specimens showed similar profiles, suggesting that the major toxin in puffer Masinloc Bay is STX (Fig. 35). The Masinloc Bay where these puffer specimens were collected is well known for the bloom of Pyrodinium bahamense var. compressum, a tropical PSP toxinsdoi:10.5047/absm.2010.00301.0001 Fig. 34. HPLC-chromatograms of a skin extract of A. mappa. (A) Analysis by HPLC specially designed for TTX. For detail conditions see Yotsu et al. (1989). (B) and (C) analysis by HPLC specially designed for PSP toxins. For detail conditions see Oshima (1995a). Reprinted from Toxicon, 38, Sato, Ogata, Borja, Gonzales, Fukuyo and Kodama. Frequent occurrence of paralytic shellfish poisoning toxins as dominant toxins in marine puffer from tropical water, 1101–1109, © 2000, Pergamon Press plc, with permission from Elsevier. © 2010 TERRAPUB, Tokyo. All rights reserved. 28 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 35. Mol% of PSP toxins to total toxin (TTX + PSP toxins) in each organ of puffer. L: liver, I: intestine, M: muscle, S: skin. Reprinted from Toxicon, 38, Sato, Ogata, Borja, Gonzales, Fukuyo and Kodama. Frequent occurrence of paralytic shellfish poisoning toxins as dominant toxins in marine puffer from tropical water, 1101–1109, © 2000, Pergamon Press plc, with permission from Elsevier. producing dinoflagellate (Montojo et al. 2006). Bivalve in the bay become toxic almost every year. Interestingly, the toxin profile of puffer was close to that of P. bahamense var. compressum and its associated bivalves. On the other hand, Zaman et al. (1997) reported that toxin profile of freshwater and/or brackishwater puffer Tetraodon cutctia and C. patoca collected from the rivers and tributary in Dhaka, Bangladesh, was also close to that of P. bahamense var. compressum, though P. bahamense var. compressum can not grow in freshwater. Thus the PSP toxins found in puffers from Masinloc Bay was not derived from P. bahamense var. compressum. The source of STXs found in the Philippine puffers is unknown. Yasumoto et al. (1981) concluded that the STXs in the xanthid crab are not derived from dinoflagellates, as no STXs were detected in bivalves living in the same area where the toxic xanthid crab is inhabiting. Instead, they detected a low level of STXs in Jania sp., a carcacious alga which is often found in the stomach of the toxic xanthid crab, and suggested that the origin of STXs in the xanthid crab is this alga (Kotaki et al. 1983). It is possible that the xanthid crab accumulates a high level of STXs by continuous ingestion of Jania sp. over doi:10.5047/absm.2010.00301.0001 a long period. Meanwhile, the xanthid crab is known to release STXs (Noguchi et al. 1985). Arakawa et al. (1998) showed that a large proportion of the STXs injected into the xanthid crab disappeared within a short period. This finding implies that the rate of release of STXs from the xanthid crab is significantly high. Therefore, it is unlikely that the xanthid crab accumulates a high level of STXs by continuous ingestion of Jania sp. with a low concentration of STX, even over a long period. Interestingly, these STX-bearing animals often possess tetrodotoxin (TTX) as well, a potent neurotoxin which binds to the same biological receptor as STXs (Kao 1966). This suggests that organisms known to possess either STXs or TTX may possess both of these toxins. This speculation is supported by the fact that a significant amount of TTX has been detected in A. tamarense, a representative dinoflagellate capable of producing STXs. During a bloom of A. tamarense, considerable amounts of TTX accumulate in shellfish together with STXs (Kodama et al. 1993). As shown in Fig. 35 the levels of TTX in the shellfish was shown to be parallel with the levels of STXs (Kodama et al. 1993). These findings indicate that TTX found in the shellfish originates from A. tamarense. Actually, a significant level of TTX was identified in cultured cells of A. tamarense (Kodama et al. 1996b). This is the first case to be shown that A. tamarense could be the causative organism for TTX-bearing animals. It is well known that TTX is produced by bacteria, although the productivity is very low (Noguchi et al. 1986; Yasumoto et al. 1986). It is noteworthy that TTXproducing bacteria and STX-producing bacteria display similar characteristics in terms of toxin productivity. The toxin productivity of TTX-producing bacteria is very low and the TTX levels in the TTX-producing bacteria increase significantly under starvation conditions (Noguchi et al. 1986; Yasumoto et al. 1986). Although the evidence is circumstantial, various phenomena suggest that these bacteria are widely distributed in natural ecosystems. The origin of the STXs in toxic animals not associated with dinoflagellates seems to be bacteria, as in the case of TTX, and the mechanism by which the organisms become toxic owing to the presence of TTX and STXs derived from bacteria seems to be similar in each instance. 14. Possible mechanism by which pufferfish become toxic: a model based on the mechanism by which accumulation of STXs occurs in dinoflagellates It is well known that wild puffer possess a potent neurotoxin named as tetrodotoxin (TTX). Generally, TTX is contained in various toxic species of puffer. However, TTX is not always contained in all the specimens of toxic species. Non-toxic specimens are often observed among the toxic species of puffer. It has been a mystery why puffer possess the potent toxin, TTX. Although the © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 reason is still not clear, it is known by fish culturists that puffer which are artificially hatched and fed an artificial diet are nontoxic. Matsui et al. (1982) demonstrated that nontoxic cultured puffer Takifugu rubripes become toxic when fed a diet containing TTX, and indicated that TTX in the puffer is an exogenous substance which originates from the diet. On the other hand, TTX has been shown to be produced by bacteria (Noguchi et al. 1986; Yasumoto et al. 1986) and thus the source of TTX in TTX-bearing animals such as puffer is considered to be bacteria. Hence, TTX of bacterial origin is considered to accumulate in the puffer via food webs (Yasumoto et al. 1992). The screening of various marine organisms for TTX revealed that this toxin is widely distributed in marine organisms, some species of which could be components of the diet of puffer (Noguchi et al. 1983; Miyazawa et al. 1986). These studies also showed that the trumpet shell Charonia sauliae accumulates TTX by ingesting the TTX bearing starfish Astropecten polyacanthus (Narita et al. 1984). However, such a case clearly supporting the food chain theory of TTX accumulation in toxic animals is rare. The distribution of toxic components of the diet of the puffer rarely overlaps with that of toxic puffer, indicating that the puffer toxin cannot be accumulated via a simple food web mechanism. On the contrary, Noguchi et al. (1987) proposed that puffer become toxic by absorbing a low level of TTX produced by intestinal bacteria over a long period. This idea seems plausible, because TTXproducing Vibrio alginolyticus is a dominant species in the intestinal bacterial flora of toxic puffer (Noguchi et al. 1987). However, Sugita et al. (1988) reported that V. alginolyticus is also a dominant member of the intesti- nal bacterial flora of nontoxic cultured puffer T. rubripes. We analyzed TTX levels in the liver, the main toxic organ of the puffer, and in the intestine of cultured puffer specimens of 60 days and 6 months old, applying a sensitive method of analysis of TTX (Sato et al. 1990). No TTX was detected in the liver of either juvenile or adult puffer specimens, whereas a low level of TTX, 0.2 mouse unit (MU)·g–1 (where one MU is a dose required to kill a 20-g male mouse within 30 min), apparently due to intestinal bacteria, was detected in the intestine of both samples. These observations show that the level of TTX in the intestine is too low for accumulation by the puffer. TTX- and STX-bearing animals have been reported to be resistant to these toxins (Koyama et al. 1983). However, these animals die when a substantial amount of the toxin (about 1000 times the lethal dose for common animals) is injected (Koyama et al. 1983). These observations show that the toxins are also toxic to these animals. Although the mechanism is not known, puffer sometimes accumulate TTX at levels much higher than their lethal dose (Kodama et al. 1984). In this regard, species of puffer are secreting this dangerous toxin into environmental water continuously (Kodama et al. 1985). As shown in Fig. 36, puffer possess developed TTX-secreting glands in the skin which consist of specific TTXsecreting cells (TTX cells) (Kodama et al. 1986). When stimulated, the puffer secretes a considerably high amount of TTX into the environment, that is, 4 mg of TTX is often secreted on a single stimulation (Kodama et al. 1985), showing that the rate of TTX secretion is significantly high. A similar phenomenon is also observed in the case of the TTX bearing newt (Shimizu and Kobayashi Fig. 36. Light micrographs of the skin of T. pardalis. (A) Longitudial section of a large alveolar secretory gland, with an opening (arrow) to the skin surface. The cytoplasm of the scretory cells (TTX cells) are almost empty. The bar equals 100 µm. (B) Epidermis, with mucous cells, sacciform cells and free TTX cells. The bar equals 10 µm. Helly’s fluid, PAS—hematoxylin stain. SG, secretory gland; E, epidermis; D, dermis; L, lumen of the gland; MC, mucous cell; FC, free TTX cell. Reprinted from Toxicon, 24, Kodama, Sato, Ogata, Suzuki, Kaneko and Aida, Tetrodotoxin secreting glands in the skin of puffer fishes. 819–829, © 1986, Pergamon Journal Ltd. with permission from Elsevier. doi:10.5047/absm.2010.00301.0001 29 © 2010 TERRAPUB, Tokyo. All rights reserved. 30 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 1983). These findings indicate that TTX-bearing animals actively release TTX from a specifically developed organ and the rate of release of the toxin is significantly high. Given that the TTX present in the puffer is accumulated via food webs, the rate of uptake of TTX should be greater than the high rate of release, that is, a considerably large amount of TTX must be supplied to the puffer continuously. This seems consistent with our results indicating that the cultured puffer T. rubripes did not accumulate TTX, although a low level was detected in the intestine. The supply of TTX from the intestinal bacteria was too low for accumulation by the puffer. Watabe et al. (1987) reported that tritium-labeled TTX injected intraperitoneally into toxic puffer is accumulated in the liver for a short period and then transported to the skin and gallbladder to be released. About 70% of the injected TTX disappeared within 6 days. We also observed that TTX injected into the nontoxic cultured puffer T. rubripes, was accumulated in the liver first, moved to the skin, and then was released into the environment (unpublished data). About 50% of the injected TTX disappeared within a few weeks. These data show that TTX taken up by the puffer is not retained for a long period because of the high rate of secretion of TTX. Although no significant amount of TTX has been detected in the diet of the puffer, some specimens of wild puffer accumulate substantial amounts of TTX (Kodama et al. 1984). These observations lead us to contend that puffer accumulate TTX not via food webs but through some other mechanism which remains unknown. Interestingly, a considerable amount of TTX was detected in the muscle of cultured puffer T. rubripes specimens 2 weeks after injection of TTX (unpublished data). Watabe et al. (1987) also observed that injected tritium labeled TTX is detected in significant concentrations in the muscle of the puffer. In contrast, no TTX is detected in the muscle of wild specimens of T. rubripes. Tani (1945) reported that the muscle of T. rubripes is nontoxic, although considerable toxicity is often detected in the liver and other organs. Based on analyses of the toxicity of various specimens, the muscle of this species has been designated as a safe food after careful removal of the organs prone to contamination with the toxin by licensed chefs in Japan. The results of these toxicity analyses indicate that natural specimens of T. rubripes never take in such an amount of TTX at once as that administered in the experiments. The results also tend to support the view that TTX in the puffer is not accumulated via food webs. As described above, we have suggested that symbiotic bacteria in dinoflagellates are involved in the production of substantial amounts of STX. Most of the toxic species of puffer possess STXs as well as TTX (Sato et al. 1993). In specimens of the freshwater puffer, Tetraodon fangi, which caused food poisoning including lethal cases in Thailand, only STX was detected as the toxic agent doi:10.5047/absm.2010.00301.0001 (Sato et al. 1997). Therefore, the approach taken in investigation of toxins from dinoflagellates was applied to puffer toxin, that is, attempts were made to isolate bacteria from the liver tissue of toxic and nontoxic specimens of puffer. Interestingly, bacteria were isolated only from toxic specimens (Kodama et al. 1995). Polyclonal antibody against the bacterium isolated from the liver reacted with an extract of the liver from which the bacterium was isolated. Electron microscopic observations also supported the view that bacteria were present in the liver cells (Fig. 37). An extract of the isolated bacteria was found to display sodium channel blocking activity and gave a TTX-like peak on HPLC analysis. These findings suggest that bacterial symbiosis in the liver cells is involved in the mechanism of production of puffer toxin as in the case of toxic dinoflagellates. Most of the bacteria isolated from the liver of the puffer were gram-positive, which are usually not found in seawater but rather in the sediments (Kodama et al. 1995). Polyclonal antibody against the bacterium isolated from the liver reacted with an extract of the liver from which the bacterium was isolated (Fig. 38). It is well known that puffer tend to hide themselves in the bottom sediment (Katayama and Fujita 1965). It seems plausible that bacterial infection may occur in this habitat of the puffer. This may explain why puffer cultured in a cage, having no contact with bottom sediments, are nontoxic. As described above, bacteria which produce STXs or STX-like substances are widely distributed in natural ecosystems. This is also true for bacteria which produce TTX or TTX-related compounds (Noguchi et al. 1987; Simidu et al. 1990). Shewanella alga, the species found to produce TTX, was shown to produce STXs as well (Doucette et al. 1988). This species shows wide phylogenetic and spatial distribution in natural ecosystems. These observations imply that most aquatic animals are exposed to these bacteria. Therefore, we screened the viscera of various common aquatic animals for the presence of STXs and TTX (Sato et al. 1993). HPLC analyses and bioassays of the toxins partially purified from the viscera showed that all species examined showed the presence of both TTX and STXs, although the level were very low (Table 4). For further confirmation, an extract of the chum salmon, Onchorhyncs keta, in which low levels of TTX, STX, and neoSTX were detected by HPLC analysis, was prepared from a large amount of the viscera (62 kg) of specimens collected at a river mouth during the homing period. A toxin with a retention time identical to that of TTX was detected in the HPLC analysis, but was lost during the purification process; however, toxins corresponding to STX and neoSTX were obtained in a highly pure state. Mass spectrometry and NMR analyses clearly showed that these toxins were STX and neoSTX (Sato et al. 1998). These findings show that TTX and STXs are not substances found only in specific organisms but are widely © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 37. Electron micrograph of hepatocytes from Takifugu poecilonotus. (a) There are three hepatocytes in the electron micrographs. Each cell contain many microorganism-like components (arrows). No abnormal bioreactions are seen in every hepatocyte. F, fat droplet. Bar = 2 µm. (b) Enlarged part of (a). Three round microorganisms are seen. These are surrounded by double membranes from the cytoplasms of the hepatocytes. Numerous ribosomes (R) and the thick septum (S) are demonstrable. Bar = 0.5 µm. Reprinted with permission from Kodama, Shimizu, Sato, Ogata and Terao. The infection of bacteria in the liver cells of toxic puffer—A possible cause for organisms to be made toxic by tetrodotoxin in association with bacteria. Harmful Marine Algal Blooms—Proceed. 6th Intern. Conference Toxic Marine Phytoplankton, Nantes, October 1993, P. Lassus et al., © Technnique et Documentation—Lavoisier, 1995. Fig. 38. Double immunodiffusion of the extracts of the liver and isolated bacteria against antibody P and puffer liver. 1 and 4: the liver extract of a toxic specimen of the pufferfish Takifugu porphyreus; 2 and 6: the extract of bacteria isolated from the liver, the concentration of the extracts is different; 3 and 5: the extract of antigenic bacteria to prepare antibody P. (a) Center: antibody P, (b): Center the serum of puffer specimen T. porphyreus from which the liver (1 and 4) and bacteria (2 and 6) were prepared. Antibody P was prepared by immunizing rabbit with homogenate of bacteria isolated from the liver of another toxic specimen of T. porphyreus. Reprinted with permission from Kodama, Shimizu, Sato, Ogata and Terao. The infection of bacteria in the liver cells of toxic puffer—A possible cause for organisms to be made toxic by tetrodotoxin in association with bacteria. Harmful Marine Algal Blooms—Proceed. 6th Intern. Conference Toxic Marine Phytoplankton, Nantes, October 1993, P. Lassus et al., © Technnique et Documentation—Lavoisier, 1995. doi:10.5047/absm.2010.00301.0001 © 2010 TERRAPUB, Tokyo. All rights reserved. 31 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 32 Table 4. Occurrence of TTX and STXs in common aquatic animals. Species Shark Lemna ditropis Part TTX Toxin composition (mg mL–1 solution*) GTX1,4 GTX2,3 2.3 0.3 2.3 0.5 neoSTX STX Int Liv 2.3 1.0 Int Liv 1.1 0.7 Conger eel Conger myriaster Vis 1.3 Saury Cololabis saira Vis 1.0 Int Liv 0.6 0.2 0.6 2.1 0.2 3.1 Int Liv 1.9 1.6 0.9 0.1 0.7 0.4 0.2 0.1 Liv 1.7 Int Liv 0.8 2.5 0.3 1.8 0.3 0.1 0.1 Crayfish Procambarus clarkii Hep 0.4 1.0 Rock crab Hemigrapsus sanguineus Hep 0.1 Rock crab Pachygrapsus crassipes Hep 0.1 Marine snail Neptunea arthritica Dig Salmon Oncorhynchus keta Cod Gadus macrocephalus Parrotfish Ypsiscarus ovifrons Skipjack Katuwonus pelamis Filefish Nevodom modestus 0.2 0.1 0.1 4.2 0.3 1.9 0.1 0.9 1.0 0.1 0.9 0.3 0.1 Int, intestine; Liv, liver; Vis, viscera; Hep, hepatopancreas; Dig, digestive gland. *1 mL solution is equivalent to 100 g of organ. This article was published in Toxic Phytoplankton Blooms in the Sea (Smayda and Shimizu, eds.), Sato, Ogata and Kodama, Wide distribution of toxins with sodium channel blocking activity similar to tetrodotoxin and paralytic shellfish toxins in marine animals, pp. 429–434, © Elsevier (1993). distributed in common aquatic animals at low levels. As shown in Table 4, no significant difference was observed in the levels of these toxins in the viscera of various common animals, indicating that these toxins are not concentrated via the food web but rather they originate from intestinal bacteria. More than 90% of the homing chum salmon returning close to their home river do not consume any diet, and thus their stomachs are empty (Ueno 1993). All of the stomachs in the viscera used in the above experiments were empty, thus the STXs found in the visdoi:10.5047/absm.2010.00301.0001 cera did not originate from the diet. These findings support the view that bacteria which produce TTX and STXs are widely distributed in natural ecosystems. 15. Hypothesis on toxin accumulation in toxic organisms As described above, bacteria which produce low levels of toxins live in cells of the puffer and in dinoflagellates which display high levels of TTX and/or © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Fig. 39. Hypothesis on the mechanism by which toxic organisms become toxic in association with infectious bacteria. STXs. Toxic shellfish also possess these bacteria in their cells, carried by the vector dinoflagellates. These bacteria grow in cells of the host organisms. The host organisms kill and digest some of the bacteria that have proliferated within the host cells and thereby maintain a suitable number of bacterial cells for their survival. That is, direct biochemical interactions between the bacteria and the host occur within the cells in the host organisms. The finding that the number of bacteria found in the host is few tends to support this idea. Based on these findings, the hypothesis shown in Fig. 39 is proposed, suggesting that TTX and STXs are not the final products of their metabolisms but are intermediate products synthesized by some bacterial substance which seems to be important for their survival. Recently, the biosynthetic pathway of STX proposed by Shimizu et al. (1984) has been confirmed with STX biosynthesis gene and biosynthetic intermediate in PSP toxins-producing cyanobacteria (Kellmann et al. 2008a, b). However, occurrence of these enzymes have not been reported in toxic dinoflagellates such as Alexandrium species. Probably, the STX synthesized by bacteria is incorporated to “unknown biomolecule” suggested in Fig. 39. When the bacteria with this unknown substance invade the toxic organisms such as dinoflagellates and puffer, a part of bacteria is digested by the defense mechanism of the host organisms. During digestion, STXs or TTX bound with the biomolecule are considered to be released. Previously, we reported that TTX and STXs are released from a nontoxic high molecular weight fraction prepared from toxic scallop digestive glands or toxic puffer livers on digestion with RNase (Kodama et al. 1982b, 1983b). These findings suggest that RNA of toxinproducing bacteria is a possible candidate of the “unknown substance” shown in Fig. 39. This idea is consistent with the observation that the toxin content of the bacteria is very low (Kodama et al. 1990b), and that toxin doi:10.5047/absm.2010.00301.0001 33 production by the bacteria increases significantly under P-limiting conditions (Doucette and Trick 1995) as well as under starved conditions (Kodama et al. 1990b). Toxic animals and dinoflagellates are hosts which possess the enzyme(s) required to digest the “unknown substance” and release the toxins. If the “unknown substance” in question is RNA, the enzyme required to digest this substance would be an RNase, an enzyme which occurs widely in living organisms. Toxic organisms might possess a specific type of RNase capable of digesting foreign RNA which contains unusual components, STXs, and/or TTX. Some strains of toxic dinoflagellates are known to be nontoxic. The RNase in these strains might lack the ability to digest foreign RNA containing unusual components which are not essential for their growth. References Anderson DM. Effect of temperature conditioning on development and germination of Gonyaulax tamarensis (Dinophyceae) hypnozygotes. J Phycol 1980; 16: 166–172. Arakawa O, Noguchi T, Onoue Y. Transformation of gonyautoxins in the xanthid crab Atergatis floridus. Fish Sci 1998; 64: 334–337. Asakawa M, Takagi M, Iida A, Oishi M. Studies on the conversion of paralytic shellfish poison (PSP) components by biochemical reducing reagents. Eiseikagaku 1987; 33: 50–55 (in Japanese). Boyer GL, Sullivan JJ, Andersen RJ, Harrison PJ, Taylor FJR. Toxin production in three isolates of Protogonyaulax sp. In: Anderson DM, White AW, Baden DG (eds). Toxic Dinoflagellates. Elsevier, New York. 1985; pp. 281–286. Boyer GL, Sullivan JJ, Andersen RJ, Taylor FJR, Harrison PJ, Cembella AD. Use of high-performance liquid chromatography to investigate the production of paralytic shellfish toxins by Protogonyaulax spp. in culture. Mar Biol 1986; 93: 361–369. Boyer GL, Sullivan JJ, Andersen RJ, Harrison PJ, Taylor FJR. Effects of nutrient limitation on toxin production and composition in the marine dinoflagellate Protogonyaulax tamarensis. Mar Biol 1987; 96: 123–128. Bricelj VM, Lee JH, Cembella AD, Anderson DM. Uptake kinetics of paralytic shellfish toxins from the dinoflagellate Alexandrium fundyense in the mussel Mytilus edulis. Mar Ecol Prog Ser 1990; 63: 177–188. Bricelj VM, Lee JH, Cembella AD. Influence of dinoflagellate cell toxicity on uptake and loss of paralytic shellfish toxins in the northern quahog Mercenaria mercenaria. Mar Ecol Prog Ser 1991; 74: 33–46. Bricelj VM, Cembella AD, Laby D, Shumway SE, Cucci TL. Comparative physiological and behavioral responses to PSP toxins in two bivalve mollusks, the softshell clam, Mya arenaria, and surfclam, Spisula solidissima. In: Yasumoto T, Oshima Y, Fukuyo Y (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996; pp. 405–408. Burke JM, Marchisotto J, McLaughlin JJA, Provasoli L. Analysis of the toxin produced by Gonyaulax catenella in axenic culture. Ann NY Acad Sci 1960; 837–842. Cembella AD, Sullivan JJ, Boyer GL, Taylor FJR, Andersen © 2010 TERRAPUB, Tokyo. All rights reserved. 34 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 RJ. Variation in paralytic shellfish toxin composition within the Protogonyaulax tamarensis/catenella species complex; red tide dinoflagellates. Biochem Syst Ecol 1987; 15: 171– 186. Chang FH, Anderson DM, Kulis DM, Till DG. Toxin production of Alexandrium minutum (Dinophyceae) from the Bay of Plenty, New Zealand. Toxicon 1997; 35: 393–409. Doucette GJ, Kodama M, Franca S, Gallacher S. Bacterial interactions with harmful algal bloom species: Bloom ecology, toxigenesis, and cytology. In: Anderson DM, Cembellak AD, Hallegraeff GM (eds). Physiological Ecology of Harmful Algal Blooms. Springer, Berlin. 1988; pp. 619–647. Doucette GJ, Trick CG. Characterization of bacteria associated with different isolates of Alexandrium tamarense. In: Lassus P, Arzul G, Denn EE-L, Gentien P, Baut CM-L (eds). Harmful Marine Algal Blooms. Paris, London. 1995; pp. 33–38. Franca S, Viegas S, Mascarenhas V, Pinto L, Doucette GJ. Prokaryotes in association with a toxic Alexandrium lusitanicum in culture. In: Lassus P, Arzul G, Erard E, Gentien P, Marcaillou C (eds). Harmful Marine Algal Blooms. Paris. 1995; pp. 45–51. Franca S, Pinto L, Alvito P, Sousa I, Vasconcelos V, Doucette GJ. Studies on prokaryotes associated with PSP producing dinoflagellates. In: Yasumoto T, Oshima Y, Fukuyo Y (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996; pp. 247–350. Franco JM, Fernandez P, Reguera B. Toxin profiles of natural population and cultures of Alexandrium minutum Halim from Galician (Spain) coastal waters. J Appl Phycol 1994; 6: 275–279. Fukazawa N, Ishimaru T, Takahashi T, Fujita Y. A mechanism of ‘red tide’ formation. I. Growth rate estimate by DCMUinduced fluorescence increase. Mar Ecol Prog Ser 1980; 3: 217–222. Fukuyo Y. Theca and cyst of Gonyaulax excavata (Braarud) Balech found at Ofunato Bay, Pacific coast of northern Japan. In: Taylor DL, Seliger HH (eds). Toxic Dinoflagellate Blooms. Elsevier, North-Holland, New York. 1979; pp. 61–64. Fusetani N, Endo H, Hashimoto K, Takahashi K. Occurrence of potent toxins in the horseshoe crab Carcinoscorpius rotundicauda. Toxicon 1982; 20: 662–664. Gaines G and Elbrahter M. Heterotrophic Nutrition. The Biology of Dinoflagellates. Botanical Monographs Volume 21, FJR Tailor (ed). Blackwell, Oxford. 1987; Chap. 6, pp. 224–268. Gallacher S, Flynn KJ, Franco JM, Brueggemann EE, Hines HB. Evidence for production of paralytic shellfish toxins by bacteria associated with Alexandrium spp. (Dinophyta) in culture. Appl Environ Microbiol 1997; 63: 239–245. Glover H, Beardall J, Morris I. Effects of environmental factors on photosynthesis patterns in Pheodacylum tricornutum (Bacillariophyceae). I. Effect of nitrogen deficiency and light intensity. J Phycol 1975; 11: 424–429. Groisman EA. Resistance to host antimicrobial peptides in necessary for Salmonella virulence. Proc Natl Acad Sci USA 1992; 89: 11939–11943. Hall S, Reichardt PB, Neve RA. Toxins extracted from an doi:10.5047/absm.2010.00301.0001 Alaskan isolate of Protogonyaulax sp. Biochem Biophys Res Commun 1980; 97: 649–653. Hansen PJ, Cembella AD, Moestrup O. The marine dinoflagellate Alexandrium ostenfeldii: paralytic shellfish toxin concentration, composition, and toxicity to a tintinned ciliate. J Phycol 1992; 28: 597–603. Harada T, Oshima Y, Yasumoto T. Structures of two paralytic shellfish toxins, gonyautoxins V and VI, isolated from a tropical dinoflagellate, Pyrodinium bahamense var. compressum. Agric Biol Chem 1982; 46: 1861–1864. Harada T, Oshima Y, Yasumoto T. Natural occurrence of decarbamoylsaxitoxin in tropical dinoflagellate and bivalves. Agric Biol Chem 1983; 47: 191–193. Hashimoto Y, Kanna K, Shiokawa A. On shell-fish poisons: II. paralytic poison (Preliminary Report). Nippon Suisan Gakkaishi 1950; 15: 771–776 (in Japanese). Horowitz, W (ed). Official Methods of Analysis, 14th edn. Procedure 18.086–18.092. Association of Official Analytical Chemists, Washington, DC. 1984. Ikeda T, Matsuno S, Sato S, Ogata T, Kodama M, Fukuyo Y, Takayama H. First report on paralytic shellfish poisoning caused by Gymnodinium catenatum Graham (Dinophyceae) in Japan. In: Okaichi T, Anderson DM, Nemoto T (eds). Red Tides: Biology, Environmental Science, and Toxicology. Elsevier, New York. 1989; pp. 411– 414. Iwasaki H. The life-cycle of Porphyra tenera in vitro. Biol Bull 1961; 121: 173–187. Iwasaki H. The physiological characteristics of neritic red-tide flagellates. In: Taylor DL, Seliger HH (eds). Toxic Dinoflagellate Blooms. Elsevier, North-Holland, New York. 1979; pp. 95–100. Jacobson DM, Anderson DM. Widespread phagocytosis of ciliates and other protists by marine mixotrophic and heterotrophic thecate dinoflagellates. J Phycol 1996; 32: 279– 285. Kao CY. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev 1966; 18: 997–1049. Katayama M, Fujita S. Ecological studies on the puffer, Fugu niphobles (Jordan et Snyder) II. Bull Facul Edu Yamaguchi Univ 1965; 15: 77–84. Kawabata T, Yoshida T, Kubota Y. Paralytic shellfish poison— I. A note on the shellfish poisoning occurred in Ofunato city, Iwate Prefecture in May, 1961. Bull Japan Soc Sci Fish 1962; 28: 344–351. Kellmann R, Michali TK, Neilan BA. Identification of a saxitoxin biosynthesis gene with a history of frequent horizontal gene transfers. J Mol Evol 2008a; 67: 526–538. Kellmann R, Mihali TK, Jeon YJ, Pickford R, Pomati F, Neilan BA. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl Environ Micro 2008b; 74: 4044–4053. Kikuchi T, Sato M, Kaga Y, Sato K, Sato S, Ogata T, Kodama M. Secondary contamination of scallops by ingestion of PSP toxins in the feces of adjacent highly toxic scallops. In: Yasumoto T, Oshima Y, Fukuyo Y (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996; pp. 413–415. Kim CH, Sako Y, Ishida Y. Variation of toxin production and composition in axenic cultures of Alexandrium © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 catenella and A. tamarense. Nippon Suisan Gakkaishi 1993; 59: 633–639. Kobayashi M, Shimizu Y. Gonyautoxin—VIII, a cryptic precursor of paralytic shellfish poisons. J Chem Soc Chem Commun 1981; 827–828. Kodama M, Ogata T. New insight into shellfish toxins. Mar Poll Bull 1988; 19: 559–564. Kodama M, Fukuyo Y, Ogata T, Igarashi T, Kamiya H, Matsuura F. Comparison of toxicities of Protogonyaulax cells of various sizes. Bull Japan Soc Sci Fish 1982a; 48: 567– 571. Kodama M, Ogata T, Takahashi Y, Niwa T, Matsuura F. Gonyautoxin associated with RNA-containing fraction in the toxic scallop digestive gland. J Biochem 1982b; 92: 105–109. Kodama M, Ogata T, Noguchi T, Maruyama J, Hashimoto K. Occurrence of saxitoxin and other toxins in the liver of the pufferfish Takifugu pardalis. Toxicon 1983a; 21: 897–900. Kodama M, Noguchi T, Maruyama J, Ogata T, Hashimoto K. Release of tetrodotoxin and paralytic shellfish poison from puffer liver by RNase. J Biochem 1983b; 93: 243–247. Kodama M, Ogata T, Kawamukai K, Oshima Y, Yasumoto T. Toxicities of muscle and other organs of five species of puffer collected from the Pacific coast of Tohoku area of Japan. Bull Japan Soc Sci Fish 1984; 50: 703–706. Kodama M, Ogata T, Sato S. External secretion of tetrodotoxin from puffer fishes stimulated by electric shock. Mar Biol 1985; 87: 199–202. Kodama M, Sato S, Ogata T, Suzuki Y, Kaneko T, Aida K. Tetrodotoxin secreting glands in the skin of puffer fishes. Toxicon 1986; 24: 819–829. Kodama M, Ogata T, Sato S. Bacterial production of saxitoxin. Agric Biol Chem 1988; 52: 1075–1077. Kodama M, Ogata T, Sato S, Sakamoto S. Possible association of marine bacteria with paralytic shellfish toxicity of bivalves. Mar Ecol Prog Ser 1990a; 61: 203–206. Kodama M, Ogata T, Sakamoto S, Sato S, Honda T, Miwatani T. Production of paralytic shellfish toxins by a bacterium Moraxella sp. isolated from Protogonyaulax tamarensis. Toxicon 1990b; 28: 707–714. Kodama M, Sato S, Ogata T. Alexandrium tamarense as a source of tetrodotoxin in the scallop Patinopecten yessoensis. In: Smayda TJ, Shimizu Y (eds). Toxic Phytoplankton Blooms in the Sea. Elsevier, New York. 1993; pp. 401–406. Kodama M, Shimizu H, Sato S, Ogata T, Terao K. The infection of bacteria in the liver cells of toxic puffer—A possible cause for organisms to be made toxic by tetrodotoxin in association with bacteria. In: Lassus P, Arzul G, Denn EE-L, Gentien P, Baut CM-L (eds). Harmful Marine Algal Blooms. Paris, London. 1995; pp. 457–462. Kodama M, Sakamoto S, Koike K. Symbiosis of bacteria in Alexandrium tamarense. In: Yasumoto T, Oshima Y, Fukuyo Y (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996a; pp. 351–354. Kodama M, Sato S, Sakamoto S, Ogata T. Occurrence of tetrodotoxin in Alexandrium tamarense, a causative dinoflagellate of paralytic shellfish poisoning. Toxicon 1996b; 34: 1101–1105. Koehn FE, Hall S, Wichman CF, Schnoes HK, Reichardt PB. doi:10.5047/absm.2010.00301.0001 35 Dinoflagellate neurotoxins related to saxitoxin: Structure and latent activity of toxins B1 and B2. Tetrahedron Letters 1982; 23: 2247–2248. Kogure K, Tamplin ML, Simidu U, Colwell RR. A tissue culture assay for tetrodotoxin, saxitoxin and related toxins. Toxicon 1988; 26: 191–197. Kopp M, Doucette GJ, Kodama M, GG, Schutt C, Medlin LK. Phylogenetic analysis of selected toxic and non-toxic bacterial strains isolated from the toxic dinoflagellate Alexandrium tamarense. FEMS Microbiol Ecol 1997; 24: 251–257. Kotaki Y, Oshima Y, Yasumoto T. Analysis of paralytic shellfish toxins of marine snails. Bull Japan Soc Sci Fish 1981; 47: 943–946. Kotaki Y, Tajiri M, Oshima Y, Yasumoto T. Identification of a calcareous red alga as the primary source of paralytic shellfish toxins in coral reefs crabs and gastropods. Bull Japan Soc Sci Fish 1983; 49: 283–286. Kotaki Y, Oshima Y, Yasumoto T. Bacterial transfromation of paralytic shellfish toxins in coral reef crabs and a marine snail. Bull Japan Soc Sci Fish 1985; 51: 1009–1013. Koyama K, Noguchi T, Uzu A, Hashimoto K. Resistibility of toxic and nontoxic crabs against paralytic shellfish poison and tetrodotoxin. Bull Japan Soc Sci Fish 1983; 49: 481– 484. Laobhripart S, Limpakarnjanarat K, Saitanu K, Sangwonoloy O, Anuchatvorakul B, Leelasitorn S, Saitanu K. Food poisoning due to consumption of the freshwater puffer Tetraodon fangi in Thailand. Toxicon 1990; 28: 1372–1375. Levasseur M, Monfort P, Doucette GJ, Michaud S. Preliminary study of bacteria as PSP producers in the Gulf of St. Lawrence, Canada. In: Yasumoto T, Oshima Y, Fukuyo Y (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996; pp. 363–366. Lim PT, Sato S, Van Thuoc C, Tu PT, Huyen NTM, Takata Y, Yoshida M, Kobiyama A, Koike K, Ogata T. Toxic Alexandrium minutum (Diophyceae) from Vietnam with new gonyautoxin analogue. Harmful Algae 2007; 6: 321– 331. LoCicero (ed). Proceedings of the First International Conference on Toxic Dinoflagellate Blooms. The Massachusetts Science and Technology Foundation, Wakefield, 1975. Loeblich LA, Loeblich AR III. The organism causing New Zealand red tides: Gonyaulax excavate. In: LoCicera VR (ed). Proceedings of the First International Conference on Toxic Dinoflagellate Blooms. The Massachusetts Science and Technology Foundation, Wakefield. 1975; pp. 207– 224. MacIsaac JJ, Grunseich GS, Glover HE, Yentsch CM. Light and nutrient limitation in Gonyaulax excavate: nitrogen and carbon trace results. In: Taylor DL, Seliger HH (eds). Toxic Dinoflagellate Blooms. Elsevier North-Holland, New York. 1979; pp. 107–110. Martins CA, Kulis D, Franca S, Anderson DM. The loss of PSP toxin production in a formerly toxic Alexandrium lusitanicum clone. Toxicon 2004; 43: 195–205. Mascarenhas V, Alvito P, Franca S, Sousa I, Gago Martinez A, Rodriguez-Vazquez JA. The dinoflagellate Alexandrium lusitanicum isolated from the coast of Portugal: Observations on toxicity and ultrastructure during growth © 2010 TERRAPUB, Tokyo. All rights reserved. 36 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 phases. In: Lassus P, Arzul G, Denn EE-L, Gentien P, Baut CM-L (eds). Harmful Marine Algal Blooms. Paris, London. 1995; pp. 71–76. Matsui T, Sato H, Hamada S, Shimizu C. Comparison of toxicity of the cultured and wild puffer fish Fugu niphobles. Bull Japan Soc Sci Fish 1982; 48: 253. Miyazawa K, Jeon JK, Maruyama J, Noguchi T, Ito K, Hashimoto K. Occurrence of tetrodotoxin in the flatworm Planocera multitentaculata. Toxicon 1986; 24: 645–650. Montojo UM, Sakamoto S, Cayme MF, Gatdula NC, Furio EF, Relox JJR, Sato S, Fukuyo Y, Kodama M. Remarkable difference in accumulation of paralytic shellfish poisoning toxins among bivalve species exposed to Pyrodinium bahamense var. compressum bloom in Masinloc bay, Philippines. Toxicon 2006; 48: 85–92. Morriss I. Nitrogen assimilation and protein synthesis. In: Stewart WDP (ed). Algal Physiology and Biochemistry. Bot. Monogr. 10, Ch. 21, pp. 583–635, Blackwell Scientific Publications, Oxford. 1974. Murano M. Paralytic shellfish poisoning in Ofunato Bay and a suspected species of plankton. Bull Plankton Soc Jpn 1975; 22: 33–38. Nakayama R, Kumagai H, Tochikura T. γ-Glutamyltranspeptidase from Proteus mirabilis: Localization and activity by phospholipids. J Bacteriol 1984; 160: 1031– 1036. Narita H, Nara M, Baba K, Ohgami H, Ai T, Noguchi T, Hashimoto K. Effect of feeding a trumpet shell, Charonia sauliae with toxic starfish. J Food Hyg Soc Jpn 1984; 25: 251–255 (in Japanese). Noguchi T, Hwang DF, Arakawa O, Sugita H, Deguchi Y, Shida Y, Hashimoto K. Vibrio alginolyticus, a tetrodotoxinproducing bacterium, in the intestines of the fish Fugu vermicularis vermicularis. Mar Biol 1987; 94: 625–630. Noguchi T, Konos S, Hashimoto Y. Identity of the crab toxin with saxitoxin. Toxicon 1969; 7: 325–326. Noguchi T, Uzu A, Koyama K, Maruyama J, Nagashima Y, Hashimoto K. Occurrence of tetrodotoxin as the major toxin in a xanthid crab Atergatis floridus. Bull Japan Soc Sci Fish 1983; 49: 1882–1892. Noguchi T, Daigo K, Arakawa O, Hashimoto K. Release of paralytic shellfish poison from the exoskeleton of a xanthid crab Zosimus aneus. In: Anderson DM, White AW, Baden DG (eds). Toxic Dinoflagellates. Elsevier, New York. 1985; pp. 293–298. Noguchi T, Jeon J, Arakawa O, Sugita H, Deguchi Y, Shida Y, Hashimoto K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xanthid crab, Atergatis floridus. J Biochem 1986; 99: 311–314. Ogata T, Kodama M, Fukuyo Y, Inoue T, Kamiya H, Matsuura F, Sekiguchi K, Watanabe S. The occurrence of Protogonyaulax spp. in Ofunato Bay, in association with the toxification of the scallop Patinopecten yessoensis. Bull Japan Soc Sci Fish 1982; 48: 563–566. Ogata T, Ishimaru T, Kodama M. Effect of water temperature and light intensity on growth rate and toxicity change in Protogonyaulax tamarensis. Mar Biol 1987a; 95: 217–220. Ogata T, Kodama M, Ishimaru T. Toxin production in the dinoflagellate Protogonyaulax tamarensis. Toxicon 1987b; 25: 923–928. doi:10.5047/absm.2010.00301.0001 Ogata T, Pholpunchin P, Fukuyo Y, Kodama M. Occurrence of Alexandrium cohorticula in Japanese coastal water. J Appl Phycol 1990; 2: 351–356. Ogata T, Koike K, Nomura S, Kodama M. Utilization of organic substances for growth and toxin production by Alexandrium tamarense. In: Yasumoto T, Oshima Y, Fukuyo Y (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996; pp. 343–346. Omura T, Onodera H, Ishimaru T, Oshima Y. Non-toxic mutational subclones in the paralytic shellfish poisoning causative dinoflagellates, Alexandrium spp. La mer 2003; 41: 86–93. Onodera H, Satake M, Oshima Y, Yasumoto T, Carmichael WW. New saxitoxin analogues from the freshwater filamentous cyanobacterium Lyngbya wollei. Nat Toxins 1997; 5: 146– 151. Oshima, Y. Post-column derivatization HPLC methods for paralytic shellfish poisons. In: Hallegraeff GM, Anderson DM, Cembella AD (eds). Manual on Harmful Marine Microalgae. IOC Manuals and Guides No. 33. Intergovernmental Oceanographic Commission, UNESCO, Paris. 1995a; 81–111. Oshima Y. Chemical and enzymatic transformation of paralytic shellfish toxins in marine organisms. In: Lassus P, Arzul G, Erard E, Gentien P, Marcaillou C (eds). Harmful Marine Algal Blooms. Lavoisier, Intercept Ltd, Paris, London. 1995b; pp. 475–480. Oshima Y, Buckley LJ, Alam M, Shimizu Y. Heterogeneity of paralytic shellfish poisons—3. New toxins from cultured Gonyaulax tamarensis cells, Mya arenaria and Saxidomus giganteus. Comp Biochem Physiol 1977; 57: 31–34. Oshima Y, Yasumoto T. Analysis of toxins in cultured Gonyaulax excavata. In: Taylor DL, Seliger HH (eds). Toxic Dinoflagellate Blooms. Elsevier, North-Holland, New York. 1979; pp. 377–380. Oshima Y, Yasumoto T, Kodama M, Ogata T, Fukuyo Y, Matsuura F. Features of paralytic shellfish poison occurring in Tohoku district. Bull Japan Soc Sci Fish 1982a; 48: 525–530. Oshima Y, Hayakawa T, Hashimoto M, Kotaki Y, Yasumoto T. Classification of Protogonyaulax tamarensis from northern Japan into three strains by toxic composition. Bull Japan Soc Sci Fish 1982b; 48: 851–854. Oshima Y, Hasegawa H, Yasumoto T, Hallegraff G, Blackburn S. Dinoflagellate Gymnodinium catenatum as the source of paralytic shellfish toxins in Tasmanian shellfish. Toxicon 1987; 25: 1105–1111. Oshima Y, Hirota M, Yasumoto T, Hallegraeff GM, Blackburn S, Steffensen DA. Production of paralytic shellfish toxins by the dinoflagellate Alexandrium minutum Halim from Australia. Nippon Suisan Gakkaishi 1989; 55: 952. Oshima Y, Sugino K, Itakura H, Hirota M, Yasumoto T. Comparative studies on paralytic shellfish toxin profile of dinoflagellates and bivalves. In: Graneli E, Sundstrom B, Edler L, Anderson DM (eds). Toxic Marine Phytoplankton. Elsevier, New York. 1990; pp. 391–396. Oshima Y, Blackburn SI, Hallegraeff GM. Comparative study on paralytic shellfish toxin profiles of the dinoflagellate Gymnodinium catenatum from three different countries. Mar Biol 1993a; 116: 471–476. © 2010 TERRAPUB, Tokyo. All rights reserved. M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 Oshima Y, Itakura H, Lee KC, Yasumoto T, Blackburn S, Hallegraeff GM. Toxin production by the dinoflagellate Gymnodinium catenatum. In: Smayda TJ, Shimizu Y (eds). Toxic Phytoplankton Blooms in the Sea. Elsevier, New York. 1993b; pp. 907–912. Prakash A. Growth and toxicity of a marine dinoflagellate, Gonyaulax tamarensis. J Fish Res Bd Can 1967; 24: 1589– 1606. Sakamoto S, Ogata T, Sato S, Kodama M, Takeuchi T. Causative organism of paralytic shellfish toxins other than toxic dinoflagellates. Mar Ecol Prog Ser 1992; 89: 229–235. Sakamoto S, Sato S, Ogata T, Kodama M. Field survey of bivalve toxicity associated with toxicity of different particle sizes in seawater. In: Smayda TJ, Shimizu Y (eds). Toxic Phytoplankton Blooms in the Sea. Elsevier, New York. 1993; pp. 913–917. Sakamoto S, Sato S, Kodama M. Formation of intermediate conjugates in the reductive transformation of gonyautoxins to saxitoxins by thiol compounds. Fish Sci 2000; 66: 136– 141. Sato S, Ogata T, Kodama M, Kogure K. Determination of paralytic shellfish toxins by neuroblast culture assay. In: Aibara K, Kumagai S, Ohtsubo K, Yoshizawa T (eds). IUPAC ’88 and ICPP ’88, Mycotoxins and Phycotoxins, Tokyo. Japanese Association of Mycotoxicology, 16–19, August, 1988, Tokyo, Japan. 1988; pp. 3–4. Sato S, Komaru K, Ogata T, Kodama M. Occurrence of tetrodotoxin in cultured puffer. Nippon Suisan Gakkaishi 1990; 56: 1129–1131. Sato S, Ogata T, Kodama M. Wide distribution of toxins with sodium channel blocking activity similar to tetrodotoxin and paralytic shellfish toxins in marine animals. In: Smayda TJ, Shimizu Y (eds). Toxic Phytoplankton Blooms in the Sea. Elsevier, New York. 1993; pp. 429–434. Sato S, Kodama M, Ogata T, Saitanu K, Furuya M, Hirayama K, Kakinuma K. Saxitoxin as a toxic principle of a freshwater puffer Tetraodon fangi, in Thailand. Toxicon 1997; 35: 137–140. Sato S, Ogata T, Kodama M. Trace amounts of saxitoxins in the viscera of chum salmon Oncorhynchus keta. Mar Ecol Prog Ser 1998; 175: 295–298. Sato S, Sakai R, Kodama M. Identification of thioether intermediates in the reductive transformation of gonyautoxins into saxitoxins by thiols. Bioorg Med Chem Lett 2000a; 10: 1787–1789. Sato S, Ogata T, Borja V, Gonzales C, Fukuyo Y, Kodama M. Frequent occurrence of paralytic shellfish poisoning toxins as dominant toxins in marine puffer from tropical water. Toxicon 2000b; 38: 1101–1109. Schantz EJ. Chemistry and biology of saxitoxin and related toxins. Ann NY Acad Sci 1986; 479: 15–23. Schantz EJ, Mold JD, Stanger DW, Shavel J, Bowden FJP, Lynch JM, Wyler RS, Riegel BR, Sommer H. Paralytic shellfish poison VI. A procedure for the isolation and purification of the poison from toxic clams and mussel tissues. J Am Chem Soc 1957; 79: 5230–5235. Schantz EJ, Ghazarossian VE, Schnoes HK, Springer JP, Pezzanite JO, Claudy J. The structure of saxitoxin. J Am Chem Soc 1975; 97: 1238–1239. Sekiguchi K, Inoguchi N, Shimizu M, Saito S, Watanabe S, Ogata T, Kodama M, Fukuyo Y. Occurrence of doi:10.5047/absm.2010.00301.0001 37 Protogonyaulax tamarensis and shellfish toxicity in Ofunato Bay from 1980—1986. In: Okaichi T, Anderson DM, Nemoto T (eds). Red Tides: Biology, Environmental Science, and Toxicology. Elsevier, New York. 1989; pp. 399–402. Sekiguchi K, Sato S, Kaga S, Ogata T, Kodama M. Accumulation of paralytic shellfish poisoning toxins in bivalves and an ascidian fed on Alexandrium tamarense cells. Fish Sci 2001a; 67: 301–305. Sekiguchi K, Sato S, Ogata T, Kaga S, Kodama M. Accumulation and depuration kinetics of paralytic shellfish toxins in the scallop Patinopecten yessoensis fed Alexandrium tamarense. Mar Ecol Prog Ser 2001b; 220: 213–218. Shimizu Y, Hsu CP. Conformation of the structure of gonyautoxins-II–V by correction with saxitoxin. J Chem Soc Chem Commun 1981; 314–315. Shimizu Y, Yoshioka M. Transformation of paralytic shellfish toxins as demonstrated in scallop homogenates. Science 1981; 212: 547–549. Shimizu Y, Hsu CP, Fallon WE, Oshima Y, Miura J, Nakanishi K. The structure of neosaxitoxin. J Am Chem Soc 1978; 100: 6791–6793. Shimizu Y, Kobayashi M. Apparent lack of tetrodotoxin biosynthesis in captured Taricha torosa and Taricha granulose. Chem Pharm Bull 1983; 31: 3625–3631. Shimizu Y, Norte M, Hori A, Genenah A, Kobayashi M. Biosynthesis of saxitoxin analogues: The unexpected pathway. J Am Chem Soc 1984; 106: 6433–6434. Silva ES. Intracellular bacteria, the origin of the dinoflagellates toxicity. In: International IUPAC Symposium on Mycotoxins and Phycotoxins. Pahotox, Lausane. 1979; p. 8. Simidu U, Kita-Tsukamoto K, Yasumoto T, Yotsu M. Taxonomy of four marine bacterial strains that produce tetrodotoxin. Int J Syst Bacteriol 1990; 40: 331–336. Singh HT, Oshima Y, Yasumoto T. Growth and toxicity of Protogonyaulax tamarensis in axenic culture. Bull Japan Soc Sci Fish 1982; 48: 1341–1343. Steidinger KA. Some taxonomic and biologic aspects of toxic dinoflagellates. In: Falconer IR (ed). Algal Toxins in Seafood and Drinking Water. Academic Press, London. 1993; pp. 1–28. Sugita H, Noguchi T, Furuta M, Harada T, Murata O, Hashimoto K, Deguchi Y. The intestinal microflora of cultured specimens of a puffer Fugu rubripes rubripes. Nippon Suisan Gakkaishi 1988; 54: 733. Sullivan JJ, Iwaoka WT, Liston J. Enzymatic transformation of PSP toxins in the littleneck (Protothaca staminca). Biochem Biophys Res Commun 1983; 114: 465–472. Syrett PJ. Nitrogen metabolism of microalgae. In: Physiological bases of phytoplankton ecology. Can Bull Fish Aquat Sci Bull 1981; 210: 182–210. Tani I. Toxicological Studies on Japanese Puffers. Teikoku Tosho, Tokyo. 1945; 103 pp. (in Japanese). Turpin DH, Dobell PER, Taylor FJR. Sexuality and cyst formation in Pacific strains of toxic dinoflagellate Gonyaulaxtamarensis. J Phycol 1978; 14: 235–238. Ueno Y. Studies on the ecology and resource of maturing chum salmon off the Pacific coast of northern Honshu. Bull Natl Inst Far Seas Fish 1993; 30: 80–206. Watabe S, Sato Y, Nakaya M, Nogawa N, Oohashi K, Noguchi © 2010 TERRAPUB, Tokyo. All rights reserved. 38 M. Kodama / Aqua-BioSci. Monogr. 3: 1–38, 2010 T, Morikawa N, Hashimoto K. Distribution of tritiated tetrodotoxin administered introperitoneally to pufferfish. Toxicon 1987; 25: 1283–1289. White AW. High toxin content in the dinoflagellate Gonyaulax excavata in nature. Toxicon 1986; 24: 605–610. White AW, Maranda L. Paralytic toxins in the dinoflagellate Gonyaulax excavata and in shellfish. J Fish Res Bd Can 1978; 35: 397–402. Wichmann CF, Niemezura WP, Schnoes HK, Hall S, Reichardt PB, Darling SD. Structure of two novel toxins from Protogonyaulax. J Am Soc Sci 1981; 103: 6977–6978. Wisessang S, Ogata T, Kodama M, Fukuyo Y, Ishimaru T, Saitanu K, Yongvanich T, Piyakarnchana T. Accumulation of paralytic shellfish toxins by green mussel Perna viridis by feeding on cultured cells of Alexandrium cohorticula isolated from the Gulf of Thailand. Nippon Suisan Gakkaishi 1991; 57: 127–131. Yasumoto T, Yotsu M. Biogenetic origin and natural analogs of tetrodotoxin. In: Keeler RF, Mandava NB, Tu AT (eds). Natural Toxins: Toxicology, Chemistry and Safety. Alaken, Fort Collins. 1992; pp. 226–233. Yasumoto T, Oshima Y, Konta T. Analysis of paralytic shell- doi:10.5047/absm.2010.00301.0001 fish toxins of xanthid crabs in Okinawa. Bull Japan Soc Sci Fish 1981; 47: 957–959. Yasumoto T, Yasumura D, Yotsu M, Michishita T, Endo A, Kotaki Y. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric Biol Chem 1986; 50: 793–795. Yoshida T, Sako Y, Uchida A, Ishida Y, Arakawa O, Noguchi T. Purification and properties of paralytic shellfish poisoning toxins sulfotransferase from toxic dinoflagellate Gymnodinium catenatum. In: Yasumoto T, Oshima Y, Fukuyo Y (eds). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. 1996; pp. 499–502. Yoshimatsu S. Life history studies on two species of Alexandrium (Dinophyceae) and three species of Raphidophyceae in Seto Inland Sea. Bull Akashiwo Res Inst Kagawa Pref 1992; 36–48. Yotsu M, Endo A, Yasumoto T. An improved tetrodotoxin analyzer. Agric Biol Chem 1989; 53: 893–895. Zaman L, Arakawa O, Shimosu A, Onoue Y. Occurrence of paralytic shellfish poison in Bangladeshi Freshwater puffers. Toxicon 1997; 35: 423–431. © 2010 TERRAPUB, Tokyo. All rights reserved.