* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 17
Survey
Document related concepts
Transcript
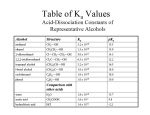
Carboxylic Acids A carbonyl with one OH attached is called a carboxylic acid One important property of carboxylic acids is the acidity O B H3C OH Acetic acid O pKa ~4-5 H3C O carboxylate Upon deprotonation a carboxylate is formed B H Carboxylic Acids Nomenclature There are two important guidelines to know about carboxylic acids: 1) The carboxylic acid has the highest priority in naming 2) In common names, the point of substitution is labeled by the Greek letter counting from the carbonyl " O OH # ! This naming is common practice amongst organic chemists, e,g, substitution at α-carbon Carboxylic Acids Examples Br O OH O OH (E)-2-pentenoic acid 3-bromo-2-methylpentanoic acid Or β-bromo-α-methylpentanoic acid (common) CO2H Cl Trans-2-chlorocyclohexanecarboxylic acid Carboxylic Acids Many carboxylic acids have a common name Aromatic rings have a number of these common names CO2H CO2H CO2H Benzoic acid Phthalic acid -As do many dicarboxylic acids O HO O OH Malonic acid (IUPAC: Propanediodic acid) Carboxylic Acids Due to the ability to resonate a lone pair of electrons on oxygen with the carbonyl, the structure of an acid has two preferred conformations O O O H O H s-cis s-trans The s-cis conformer also allows an acid to form a dimer in solution with two hydrogen bonds O H O O H O This hydrogen bonding causes a higher melting point and boiling point compared to compounds of similar molecular weight Carboxylic Acids As noted in the name, carboxylic acids are relatively acidic organic compounds The acidity is rationalized by the ability to resonate the anion after deprotonation between the two oxygen atoms H3C O O O H3C OH O H3C O The large dipole of the carbonyl though also stabilizes the anion formed after deprotonation O H3C ≅ OH O !!+ H3C OH O !!+ H3C O Anion stabilized by adjacent partial positive charge Carboxylic Acids In addition to being acidic, however, carboxylic acids can also be protonated to act as a Lewis base O H3C O H+ H3C OH H O OH H3 C O H H The question is which oxygen becomes protonated preferentially When the carbonyl oxygen is protonated, the charge can be delocalized through resonance O H3C H OH O H3 C H OH O H3C H O O H H3 C O H H O !!+ H ≅ H3 C O H When the hydroxyl oxygen is protonated, however, resonance cannot occur (already have 8 electrons in outer shell) and the cation is destabilized by adjacent partial charge Therefore carbonyl oxygen is protonated preferentially Synthesis of Carboxylic Acids Oxidative Routes H2CrO4 OH Other Routes O OH H2SO4 1) O3 H2O CN H+, ! OH 1) Mg Br H2O2 H 2) CO2 O OH O OH KMnO4 OH O 2) H2O2 O O CO2H Thus a variety of alcohols, alkenes, alkyl halides, alkyl substituted aromatic rings, aldehydes can be converted into carboxylic acids Reactions of Acyl Compounds There is a commonality amongst carbonyl reactions, they have a nucleophile react at the carbonyl carbon O O NUC H3 C X H3 C O X NUC H3 C NUC X Generate a tetrahedral intermediate that can expel a leaving group O H3 C O NUC H3 C H With ketones and aldehydes, however, there was no suitable leaving group present H NUC In acidic conditions, have same mechanism but first protonate carbonyl to make a more electrophilic carbon O H3 C O H+ X H3C H X O NUC H3 C H X NUC O H3 C NUC X Fischer Esterification One common reaction is to convert a carboxylic acid into an ester (called Fischer esterification) O H3C O H+ H3C OH H O ROH OH H OH OR H H3C Protonate carbonyl -H+ O H3C H H -H2O OR H3C O H OH OR O H+ H3C H OH OR -H+ O H3C OR Overall converted acid into ester, each step is an equilibrium Emil Fischer (1852-1919) Also invented “Fischer Projections” Esterification Other methods to synthesize an ester from a carboxylic acid include: SN2 reaction with carboxylate nucleophile O H3C O base H3C OH O CH3Br O H3C OCH3 Diazomethane O H3C O H2 C N N OH H3C O H3 C N N O H3C OCH3 Yields are quite high, but reaction is potentially dangerous for most lab-scale reactions a different route to esters is preferred Amides Direct acid to amide interconversion An amide can also be formed directly from carboxylic acid by combining the two reagents O H3C O OH RNH2 H3C O ! O RNH3 H3 C NHR H2 O The first step is merely an acid/base reaction as the amine base abstracts the acidic hydrogen To form the amide bond, the ionic salt needs to be heated This procedure is not as mild as a Fischer esterification, need to heat the reaction (usually >100 ˚C) to drive off water -Loss of water as steam shifts equilibrium to products Amides Since the direct conversion of an acid and amine to an amide requires high heat, a more efficient method to convert the reagents was developed The key is activating the acid into a better leaving group that doesn’t have a labile hydrogen R O H3C OH Because of diimide, carbon is very electrophilic H3 C R O N C NHR N C N R There are many variants of diimide coupling reagents by changing R group O O RNH2 NHR H3 C R O N C NHR The amine can react at activated carbonyl with a stable leaving group The leaving group is protonated to form a stable urea complex O H3 C O R N H N H R R NHR O N C NHR Acid Chlorides Carboxylic acid can be converted into acid chlorides by reaction with thionyl chloride O H3C O SOCl2 OH H3C Cl The mechanism once again involves converting the acid into a better leaving group O H3C OH Cl O S O Cl H3 C SOCl2 O HCl H3C -SO2 Cl After deprotonation obtain acid chloride O S O Cl Cl H HO Cl O S H3C O Cl O H3 C H H3C O O O O S O S Cl Cl HCl Cl Anhydrides Acid anhydrides (often just shortened to anhydrides) literally means loss of water from acid O H3C O OH H3C O OH H3 C O O CH3 H2O Typically need to again make acid a better leaving group for reaction to proceed Use acid chlorides O H3C O O SOCl2 OH H3C H3C O OH Cl H3C O O H3 C O CH Cl 3 H O O Or DCC coupling O H3C O OH H3C O DCC OH H3 C O O urea CH3 CH3 HCl Ketones While a Fischer esterification is an example of reacting a carboxylic acid in acidic conditions by first protonating the carbonyl, most basic nucleophiles will simply deprotonate the carboxylic acid O H3C O NUC NUC H H3C OH O The carboxylate is less electrophilic that either a ketone or aldehyde and thus it is more difficult to react with nucleophiles and often this is the final product before work-up Organolithiums, however, are much stronger nucleophiles and are able to react O H3C O RLi OH H3C RLi O O O H3C H+ R The dianion has no leaving group O HO OH -H2O H3C R H3C R After protonation, the geminal diol equilibrates to ketone Overall acids can be converted to ketones with two equivalents of organolithium Alcohols Another nucleophilic reaction that can occur with carboxylate anions is reaction with LAH O H3C O LAH OH H3C H3Al H O Li H3C O AlH2 O H O H3 C O AlH2 H 1) LAH 2) H2O Initially an aldehyde is generated, but it occurs in the presence of LAH and thus it reacts a second time to yield an alcohol H H H3C OH NaBH4 is not a strong enough hydride source for reaction, need to use LAH O OH 1) LAH 2) H2O OH Alcohols In addition to using the metal hydrides as a reducing agent, carboxylic acids have also been found to be reduced to an alcohol using borane 1) BH3•THF 2) H2O O H3 C OH H H H3C OH What is unique about this reduction compared to lithium aluminum hydride is that the carboxylic acid is reduced faster than other carbonyls This leads to selectivity differences in compounds with multiple carbonyls O H3C 1) LAH 2) H2O O OH OH H3 C OH NaBH4 H3C 1) BH3•THF 2) H2O OH LAH is unselective OH NaBH4 does not reduce acids OH Borane reduces acids first O O H3C Decarboxylation If a carboxylic acid is β to another carbonyl, then the acid will decarboxylate readily under gentle heating O H3C rotate carbon-carbon bond O OH O H3 C H O O ! H H3 C O The enol formed after losing carbon dioxide equilibrates to a ketone O C O O H3C CH3 Anytime a carboxylic acid is β to any type of carbonyl, a decarboxylation can occur O HO O O ! OH HO CH3 O C O Decarboxylation A similar decarboxylation occurs with carbonic acid and carbamic acid derivatives O RO O H ROH O C O ROH2 O C O NH3 RO Monoester of carbonic acid O H2 N O H Carbamic acid If the acid functionality is protected as an ester, then the decarboxylation cannot occur RO O O O OR Carbonates H2 N OR Carbamates H2 N NH2 Urea Hunsdiecker Another type of decarboxylation involves an electrochemical oxidation of a carboxylate to a carboxyl radical which subsequently loses carbon dioxide O O R H O H3C O electrochemical O M R O -CO2 R R Called Kolbe electrolysis R A similar decarboxylation occurs with “Hunsdiecker” reaction where an alkyl bromide is formed from carboxylic acids O O H3C O Br2 O Ag R O AgBr -CO2 Br R O R O R Br R Generates an alkyl bromide from carboxylic acid, carboxyl radical propagates reaction O