* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Download PDF
Extracellular matrix wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
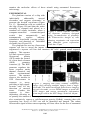
MEASURING THE IMPACT OF DIELECTROPHORESIS ON CELL PHYSIOLOGY USING A HIGH-CONTENT SCREENING PLATFORM Salil P. Desai and Joel Voldman Massachusetts Institute of Technology, Cambridge, MA, USA ABSTRACT Here we show the design and implementation of a microfabricated platform for screening the effects of electric fields on mammalian cells. Specifically, we have screened for electric field conditions that are commonly used in the manipulation of cells using dielectrophoresis. To assay the physiological state of the cells, we constructed and cloned a stress-reporting cell line. Results obtained with this platform indicate that both high field strengths and low-frequency fields adversely affect cell physiology. KEYWORDS: Dielectrophoresis, stress, physiology, high-content screening. INTRODUCTION Dielectrophoresis (DEP) is an important technique for the manipulation of cells [1]. Although researchers have studied the gross effects of DEP on cell viability and growth [2], little is known about how electric fields couple through intracellular signaling pathways to alter cell physiology. It is well known that electric fields used for DEP can not only stress cells via temperatures rises due to Joule heating of the culture media, but can also potentially directly interact with cells (e.g., via voltage-gated ion channels) [3]. Consequently, it is imperative that we understand the effects of DEP manipulation on cell physiology to determine whether DEP manipulation itself can alter particular phenotypes of interest and confound downstream biological assays. To this end, we have developed a microfabricated, highcontent screening platform that can apply a large number of different electrical stimuli to cells and then Figure 1: Device design and E-field generation. (A) Schematic (not to scale) of top-view of an array of 16 electrode sites. (B) Technique for preparing device for screen. (C) Images of electrode chips (left), bottom electrode (inset) and packaging setup (right). (D) A customdesigned computer-controlled relay box switches sinusoidal waveforms from a function generator to each individual electrode site. Twelfth International Conference on Miniaturized Systems for Chemistry and Life Sciences October 12 - 16, 2008, San Diego, California, USA 978-0-9798064-1-4/µTAS2008/$20©2008CBMS 1308 monitor the molecular effects of those stimuli using automated fluorescence microscopy. EXPERIMENTAL The platform consists of a chip with individually addressable arrayed electrodes and support electronics to generate the desired waveforms (Figure 1A, C). Mammalian cells are seeded on the chip (Figure 1B) and then the entire assembly is clamped and placed in a standard cell culture incubator, where a Figure 2. Stress reporter cell line. computer-controlled custom-designed (A) Reporter construct designed switch box automatically and using co-transfection of plasmids. autonomously applies arbitrary (B) Fluorescence images of cells stimulation waveforms (varying voltage, showing constitutive red expression frequency, and duration) to individual and inducible green expression. electrode sites (Figure 1D). Scale bar 25 μm. The platform can use any fluorescent reporter cell line to assess the impact of the applied fields. We specifically engineered a reporter cell line that monitors the cells’ general stress response pathway. This reporter cell line expresses green fluorescent protein (GFP) under the control of a heat shock element (HSE) promoter construct [4]. The HSE promoter regulates the stress response under thermal, chemical, and other environmental stresses. The reporter shows a 10× increase in GFP fluorescence in response to stress, Figure 3. Imaging and assay readout. Composite providing a high signalimage (left) showing multiple images from a single to-noise ratio for the electrode. Via multi-wavelength fluorescence imaging detection of stressed of each electrode, we use DsRed images to mask the states. Further, the GFP images and extract quantitative information response is dosefrom single cells in the GFP image. Scale bar 40 μm. dependent, allowing us to quantify the amount of stress by the fluorescence intensity. These reporter cells also constitutively express a red-fluorescent protein (DsRed), ensuring that cells expressing low levels of GFP can still be identified and imaged. The robust fluorescence signal of these stress-reporting cell lines allow for the use of automated Twelfth International Conference on Miniaturized Systems for Chemistry and Life Sciences October 12 - 16, 2008, San Diego, California, USA 1309 microscopy techniques for acquiring data (Figure 3) and the use of image processing algorithms to extract quantitative information from single cells. RESULTS AND DISCUSSION We have used this system to assess the stress response of cells to varying stimulus voltages, frequencies, and durations (Figure 4). The voltage sweep shows Figure 4. Effects of Electric fields. (A) The voltage sweep shows a dramatic increase in cellular stress with increase in voltage. (B) A frequency sweep indicating that cells are stressed at low frequencies (possibly due to transmembrane loading effects). (C) A heat map showing a voltage sweep for different durations of field exposure. Longer durations of exposure show increased cellular stress levels. an increase in stress with voltage, which is to be expected as increasing voltage will increase heating and thus thermal stress. The frequency sweep shows a slight decrease with increasing frequency, perhaps due to lower transmembrane potentials at higher frequencies. Finally, the voltage-vs.-duration heat map shows that one can trade off higher voltage for shorter duration to exact the same stress. CONCLUSION This platform is the first system that enables the quantification of the impact of a wide range of electrical field strengths, frequencies and durations on cell physiology at a molecular level. With this platform we can provide both maps of physiologically optimal conditions for DEP manipulation and probe the fundamental biological processes that are modulated by electrical fields. REFERENCES [1] Voldman, J. Electrical forces for microscale cell manipulation. Annu. Rev. Biomed. Engr., 8, 425- 454 (2006). [2] Fuhr, G., Glasser, H., Muller, T. & Schnelle, T. Cell manipulation and cultivation under AC electric-field influence in highly conductive culture media. Biochim. Et Biophy. Acta, 1201, 353-360 (1994). [3] Marszalek, P., Liu, D. S., and Tsong, T. Y. Schwan equation and transmembrane potential induced by alternating electric fields, Biophys. J., 58, 1053-1058 (1990). [4] Vasanawala, F., Tsang, T., Fellah, A., Yorgin, P., and Harris, D.T. A novel expression vector induced by heat, γ-radiation and chemotherapy, Gene Ther. Mol. Biol., 5, 1-8 (2000). Twelfth International Conference on Miniaturized Systems for Chemistry and Life Sciences October 12 - 16, 2008, San Diego, California, USA 1310