* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download xia immune activation 1
Lymphopoiesis wikipedia , lookup
Molecular mimicry wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Major urinary proteins wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Innate immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
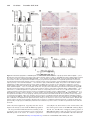
Vascular Medicine Immune Activation Resulting From NKG2D/Ligand Interaction Promotes Atherosclerosis Mingcan Xia, MD, MS; Nadia Guerra, PhD; Galina K. Sukhova, PhD; Kangkang Yang, PhD; Carla K. Miller, RD, PhD; Guo-Ping Shi, DSc; David H. Raulet, PhD; Na Xiong, PhD Background—The interplay between the immune system and abnormal metabolic conditions sustains and propagates a vicious feedback cycle of chronic inflammation and metabolic dysfunction that is critical for atherosclerotic progression. It is well established that abnormal metabolic conditions, such as dyslipidemia and hyperglycemia, cause various cellular stress responses that induce tissue inflammation and immune cell activation, which in turn exacerbate the metabolic dysfunction. However, molecular events linking these processes are not well understood. Methods and Results—Tissues and organs of humans and mice with hyperglycemia and hyperlipidemia were examined for expression of ligands for NKG2D, a potent immune-activating receptor expressed by several types of immune cells, and the role of NKG2D in atherosclerosis and metabolic diseases was probed with the use of mice lacking NKG2D or by blocking NKG2D with monoclonal antibodies. NKG2D ligands were upregulated in multiple organs, particularly atherosclerotic aortas and inflamed livers. Ligand upregulation was induced in vitro by abnormal metabolites associated with metabolic dysfunctions. Using apolipoprotein E– deficient mouse models, we demonstrated that preventing NKG2D functions resulted in a dramatic reduction in plaque formation, suppressed systemic and organ inflammation mediated by multiple immune cell types, and alleviated abnormal metabolic conditions. Conclusions—The NKG2D/ligand interaction is a critical molecular link in the vicious cycle of chronic inflammation and metabolic dysfunction that promotes atherosclerosis and might be a useful target for therapeutic intervention in the disease. (Circulation. 2011;124:2933-2943.) Key Words: atherosclerosis 䡲 immune system 䡲 inflammation 䡲 liver 䡲 NKG2D A long with other risk factors, such as metabolic dysfunction, immune cells are critically involved in promoting atherosclerosis.1,2 Abnormal metabolic conditions induce stress responses in cells of artery walls to initiate vascular inflammation and result in the recruitment of immune cells that enhance the inflammation and promote plaque formation. Conversely, chronic inflammation exacerbates metabolic dysfunction, creating a vicious cycle of chronic inflammation and metabolic dysfunction that propagates atherosclerosis.3 Editorial see p 2809 Clinical Perspective on p 2943 Macrophages are the most abundant immune cells in plaques,1 where they accumulate lipid contents and secrete proinflammatory cytokines and chemokines. Various lymphocytes, including T and natural killer (NK) cells, are also detected in plaques and involved in atherosclerosis.4 – 6 A role for T/B cells in atherosclerosis was initially suggested by the findings that chow-fed Rag1⫺/⫺apolipoprotein E– deficient (ApoE⫺/⫺) mice, which lack T/B cells, developed atherosclerotic lesions more slowly than did ApoE⫺/⫺ control mice.7 Subsequent experiments suggested a role for CD4⫹ T cells in promoting the disease by responding to antigens associated with abnormal metabolites and enhancing production of interferon-␥ (IFN-␥).8 Additional studies found a modest decrease in plaque formation in mice lacking NKT cells, a T-cell subset reactive against specific lipids.6,9 On the other hand, regulatory T cells inhibit atherosclerosis by suppressing immune activation.10 Innate lymphocytes such as NK cells have also been implicated in promoting atherosclerosis.6,11 Involvement of immune cells in atherosclerosis is thought to be initiated by abnormal metabolic conditions and the Received March 29, 2011; accepted September 27, 2011. From the Center of Molecular Immunology and Infectious Diseases, Departments of Veterinary and Biomedical Sciences (M.X., K.Y., N.X.) and Nutrition (C.K.M.), Pennsylvania State University, University Park; Department of Molecular and Cell Biology, University of California, Berkeley (N.G., D.H.R.); and Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (G.K.S., G.S.). Current address for N.G. is Division of Cell and Molecular Biology, Imperial College, London, UK. Current address for C.K.M. is Department of Human Nutrition, Ohio State University, Columbus. Guest Editor for this article was Donald D. Heistad, MD. The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA. 111.034850/-/DC1. Correspondence to Na Xiong, PhD, Department of Veterinary and Biomedical Sciences, Pennsylvania State University, 115 Henning Bldg, University Park, PA 16802. E-mail [email protected] © 2011 American Heart Association, Inc. Circulation is available at http://circ.ahajournals.org DOI: 10.1161/CIRCULATIONAHA.111.034850 Downloaded from http://circ.ahajournals.org/ at2933 UNIV OF CALIFORNIA LIB on January 29, 2013 2934 Circulation December 20/27, 2011 resulting stress to arterial endothelial cells. Abnormal metabolic components activate endothelial cells and macrophages of susceptible regions such as aortic arches through Toll-like receptors 2/4 to promote vascular inflammation and atherosclerosis.12,13 Stressed endothelial cells also upregulate chemokines and adhesion molecules (including CCL5, CXCL1, macrophage migration inhibitory receptor, E-selectin, and vascular cell adhesion molecule 1) to recruit atherogenic immune cells that promote vascular inflammation (reviewed in Rao et al14). Immune cells, including NK and NKT cells, are also activated in other inflamed organs, such as the liver, to contribute indirectly to the atherosclerosis by increasing systemic inflammation and metabolic dysfunction. However, molecular events that link activation of different immune cells and abnormal metabolic conditions in atherosclerotic progression are not well understood. NKG2D (also called killer cell lectin-like receptor subfamily K member 1 or KLRK1) is a potent activating receptor expressed by several types of immune cells involved in atherosclerosis, including NK, NKT, ␥␦ T, and subsets of activated ␣ T cells.15,16 Multiple different membrane proteins have been identified as ligands for NKG2D. In humans, they include 2 major histocompatibility complex class I chain–related family members (MICA and MICB) and 5 UL16-binding protein family members (ULBP1-4 and RAET1G). In mice, they include 5 retinoic acid early transcripts 1 family members (Rae-1␣, , ␥, ␦, and ⑀), 3 histocompatibility 60 family members (H60a, H60b, and H60c), and MULT-1 (UL16-binding protein-like transcript 1).15 These ligands are generally expressed at low levels or not at all on healthy cells but are upregulated under various pathological conditions such as tumorigenesis, viral infection, and genotoxic stress. Interaction of the ligands with NKG2D stimulates or costimulates the NKG2D-expressing immune cells to proliferate, produce cytokines (including IFN-␥, granulocyte-macrophage colony-stimulating factor, macrophage inflammatory protein 1-␣, and interleukin-2), and/or lyse target cells that express the ligands.15–20 Although the upregulation of NKG2D ligands plays an important role in host defense against tumors and viral infections,19,21 their abnormal upregulation could contribute to immune-mediated inflammatory diseases such as arthritis, celiac disease, and type 1 diabetes mellitus.22–25 We report herein that the NKG2D ligands were upregulated in humans and mice with hyperglycemia and/or hyperlipidemia and play a critical role in atherosclerosis, liver inflammation, and associated metabolic disease. Methods Mouse Models and Human Samples ApoE⫺/⫺ mice on the C56BL/6 background were purchased from Jackson Laboratory. NKG2D knockout mice (Klrk1⫺/⫺ mice) on the C56BL/6 background were described recently.26 ApoE⫺/⫺ and Klrk1⫺/⫺ mice were intercrossed to generate Klrk1⫺/⫺ApoE⫺/⫺ mice. The Klrk1 and ApoE knockout genotypes were determined by genomic polymerase chain reaction, as exemplified in Figure I in the online-only Data Supplement. In the Western diet (WD)–accelerated model of atherosclerosis, male ApoE⫺/⫺ and Klrk1⫺/⫺ApoE⫺/⫺ mice were switched from normal chow to a high-fat diet (35.5% Fat, Bio Serv) at the age of 6 weeks and analyzed for plaques, blood cytokines, and metabolites 8 to 9 weeks later. In the streptozotocin-induced diabetic model of atherosclerosis,27 6-week-old male ApoE⫺/⫺ and Klrk1⫺/⫺ApoE⫺/⫺ mice were injected intraperitoneally with streptozotocin (55 mg/kg in 0.05 mol/L citrate buffer, pH 4.5) daily for 6 days. The treated mice were confirmed diabetic (blood glucose ⬎350 mg/dL) and fed on chow for an additional 8 to 9 weeks before analysis. In both models, the Klrk1⫺/⫺ApoE⫺/⫺ and ApoE⫺/⫺ mice were always treated and analyzed in parallel. In experiments to test the effect of NKG2D antibody blockade on atherosclerosis, 6-week-old male ApoE⫺/⫺ mice were injected intraperitoneally with monoclonal anti-NKG2D (clone MI-6, rat IgG2a)16 or isotype-matched control antibodies 1 week and 3 days before the streptozotocin treatment and weekly afterward for 8 weeks (200 g for the first injection and 100 g for subsequent injections). The mice were analyzed 8 to 9 weeks after the beginning of streptozotocin treatment. All procedures were approved by the Institutional Animal Care and Use Committee of Pennsylvania State University. Use of biosamples of humans was approved by the institutional review boards of Pennsylvania State University and Harvard Medical School. Antibodies and Flow Cytometry The NKG2D antibody (MI-6) was prepared in house. Pan-Rae-1 antibody was purchased from R&D Systems. PE-anti-CD31, PECy5CD11b, PECY7-anti-CD3, PE-anti-TCR, PE-anti-F4/80, and Alexa647-anti-NKG2D antibodies were purchased from eBioscience. FITC-anti-NK1.1, PECy5-anti-TCR  , Alexa488-antiinterleukin [IL]-6, PE-anti-IL-4, and PECY7-anti-IFN-␥ antibodies and the mouse IL-6 enzyme-linked immunosorbent assay set were from BD Bioscience. For flow cytometry, cells were stained with the proper combination of antibodies (indicated in the figure) and analyzed on a flow cytometer FC500 (Beckman Coulter). En Face Oil Red O Staining and Quantification of Plaque Formation Aortas beginning at the root outside the heart and stopping at the thoracic diaphragm were collected, opened up longitudinally, fixed in 10% formalin, and stained with Oil red O dye as described.12 Sizes of total aortic arch and neighboring regions and of Oil red O–staining plaque areas within the regions were calculated on the basis of digital pictures of the stained aortas with the use of Adobe Photoshop, as reported.28 The extent of plaque formation was expressed as the percentage of Oil red O–stained area of the total area of the aortic arch and neighboring region. Immunohistochemical Staining Cryosections of mouse atherosclerotic plaques were stained with polyclonal goat anti-mouse Rae-1 or H60 antibody (R&D Systems or Santa Cruz Biotechnology, respectively). Cryosections of human aortic plaques were stained with monoclonal anti-MICA/B (clone 6D4, eBioscience), anti-CD68, or anti-CD31 antibodies, performed in a manner similar to that described previously.29 Cellular Isolation Mononucleocytes, endothelial cells, and hepatocytes were isolated from aortas or livers according to published procedures.30,31 Briefly, the liver was perfused with 3 mL 50 U/mL heparin (Sigma-Aldrich) and 3 mL 0.05% collagenase A/0.05% Dispase II (Roche) via the portal vein and then pressed through a cell strainer (PGC Scientific). Liver mononucleocytes and hepatocytes were separated by 40%/80% Percoll solution (Sigma-Aldrich). For isolation of cells from normal aortas and atherosclerotic aortic plaques, mouse aortas were first perfused with 50 U/mL heparin and then digested with an enzyme cocktail dissolved in phosphate-buffered saline (125 U/mL collagenase type XI, 60 U/mL hyaluronidase type I-s, 60 U/mL DNase1, 450 U/mL collagenase type I, and 20 mmol/L Hepes, Sigma-Aldrich) at 37°C for 1 hour. The isolated cells were counted and used for analyses. Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Xia et al NKG2D Promotes Atherosclerosis 2935 Figure 1. Upregulation of NKG2D ligands in sera and aortic plaques of patients with type 2 diabetes mellitus. A, A high percentage of patients with type 2 diabetes mellitus have elevated levels of soluble major histocompatibility complex class 1–related chain A (MICA) in the sera. The dashed line indicates the detection limit. One dot represents 1 sample, and the short flat lines indicate average concentrations. Fisher exact test was used to compare proportions of healthy vs diabetic people with detectable MICA expression. *P⬍0.05, **P⬍0.01, ***P⬍0.001 (applied for all figures). B through J, Detection of MICA/B proteins by immunohistochemical staining on human aortic plaques. Cryosections of the plaques were stained with anti-MICA/B antibodies (B, D, F, H), whereas adjacent sections were stained with anti-CD68 or CD31 antibodies to reveal macrophages or endothelial cells (C, E, G, I). A section of normal aorta was used as a control (J). Real-Time Reverse Transcription Polymerase Chain Reaction Analysis RNA was analyzed for transcripts of Rae-1␦, Rae-1⑀, and H60b by quantitative real-time reverse transcription polymerase chain reaction, as described previously.18,19,32 In Vitro Treatment of Macrophages Resident cells were flushed out of the peritoneal cavity of naive C56BL/6 mice with phosphate-buffered saline and plate-adhered overnight. The adherent macrophages were cultured in 10% fetal bovine serum–supplemented Dulbecco’s modified Eagle’s medium containing 10 g/mL human oxidized low-density lipoprotein (LDL) (medium-oxidized with copper [II] sulfate), natural LDL (Kalen Biomedical), 200 g/mL advanced glycation end products (Fitzgerald Industries International), or lipopolysaccharides for 2 days33 and then analyzed for expression of NKG2D ligands. Western Blot Analysis of Total Rae-1 Proteins The macrophages were lysed, separated on polyacrylamide gel electrophoresis gels, transferred to polyvinylidene fluoride membrane, blotted with anti-Rae-1 antibody (C20, Santa Cruz Biotechnology), and developed with an enhanced chemiluminescence system (GE Healthcare Life Sciences). Multiplex Analysis of Serum Cytokines Mouse sera were analyzed directly on a mouse proinflammatory7plex kit (IL-6, tumor necrosis factor-␣, IFN-␥, IL-12p70, IL-10, IL-1, and KC/CXCL1) (Meso Scale Discovery, Gaithersburg, MD). Assessment of Cholesterol and Triglyceride Levels in Serum Levels of cholesterol and triglycerides were analyzed on a Vet Test Chemistry Analyzer (IDEXX Laboratories, Inc, Maine). Alanine Aminotransferase Activity Analysis Alanine aminotransferase activities in the serum were determined with the use of a kit from Bioo Scientific (Austin, TX) according to the manufacturer’s instruction. Ex Vivo Culture of Liver Explants Phosphate-buffered saline–perfused livers were excised from WDfed ApoE⫺/⫺ or Klrk1⫺/⫺ApoE⫺/⫺ mice. Equal weights of excised livers were cut into ⬇1-mm3 cubes and cultured in media for 1 or 3 days. The culture media were recovered and analyzed for cytokines by enzyme-linked immunosorbent assay. Intracellular Cytokine Staining Mononucleocytes isolated from livers were cultured in media overnight in the presence of brofeldin A and analyzed by intracellular cytokine staining for the production of IL-6 and IFN-␥ in gated subsets of immune cells according to the manufacturers’ instructions (eBioscience and Biolegend). Enzyme-Linked Immunosorbent Assay Detection of Soluble MICA MICA proteins in sera were assayed with the use of an enzymelinked immunosorbent assay kit (R&D Systems, Minneapolis, MN). Statistical Analyses Data are expressed as mean⫾SE. Two-tailed Student t tests were used to determine statistical significance for 2-group comparisons. The ANOVA test was used for analysis of combined results of repeated experiments or multiple-group comparison (with Tukey adjustment). The Fisher exact test was used to compare proportions of diabetic and healthy people with detectable levels of soluble MICA proteins (sMICA) in sera. P⬍0.05 is considered significant. Results Upregulation of NKG2D Ligands in Sera and Atherosclerotic Plaques of Humans With Metabolic Dysfunction To investigate whether NKG2D or its ligands are involved in atherosclerosis associated with metabolic dysfunction, we first sought evidence that NKG2D ligands were upregulated in patients with type 2 diabetes mellitus (Table I in the online-only Data Supplement) by assessing sMICA in their sera. sMICA are enzymatically cleaved products of membrane MICA and were detected abundantly in patients with various cancers and immune disorders, but not healthy people.22,34,35 Of 22 patients with diabetes mellitus tested, 45% (10) had detectable sMICA (⬎25 pg/mL), and 27% (6) had high levels (400 –12250 pg/mL), whereas in the healthy controls, only 11% (2/19) had detectable levels, and 5% (1/19) had high levels of sMICA in sera (Figure 1A). These results suggest that a high percentage of patients with type 2 diabetes mellitus have upregulated MICA expression. We tried to associate the sMICA detection in the patients with other parameters (ages, years with diabetes mellitus, and glucose levels) but found no correlation (Table I in the Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 2936 Circulation December 20/27, 2011 Figure 2. Preferential upregulation of NKG2D ligands in atherosclerotic aortas and livers of apolipoprotein E– deficient (ApoE⫺/⫺) mice. A, Real-time reverse transcription polymerase chain reaction analysis of Rae-1␦, Rae-1⑀, and H60b transcripts in RNA samples isolated from aortic arch regions of Western diet (WD)–fed ApoE⫺/⫺ mice. Samples of age-matched WD- and chow-fed wild-type (WT) mice were used as controls. The experiment included 3 mice per group and was representative of 2 repeated experiments of a total of 6 mice. B, Immunohistochemical staining of sections of atherosclerotic aortic arch regions (top and middle) and “plaque-free” thoracic regions (bottom) of the same WD-fed ApoE⫺/⫺ mice with Rae-1 (left) or H60 antibodies (right). The pictures in the middle are higher amplifications (⫻400) of the boxed areas of the top panels (⫻100). C, Flow cytometry of cell surface Rae-1 expression on macrophages (gated on the CD45⫹CD11b⫹F4/80⫹ population) and endothelial cells (CD45⫺CD31⫹ population) isolated from atherosclerotic aortas of WD-fed ApoE⫺/⫺ mice or normal aortas of WT mice. The gray areas indicated isotype-matched control antibody staining. D, Real-time reverse transcription polymerase chain reaction analysis of Rae-1␦ transcripts in indicated organs of WD-fed ApoE⫺/⫺ mice and WT mice. ***Statistical significance compared with values of corresponding tissues of WT mice; n⫽4. E, Real-time reverse transcription polymerase chain reaction analysis of Rae-1␦ in purified macrophages of livers of WD-fed ApoE⫺/⫺ mice and WT mice; n⫽2. F and G, Flow cytometry of Rae-1 expression on CD11b⫹F4/80⫹ liver macrophages (F) and hepatocytes (G) of WD-fed ApoE⫺/⫺ mice. The experiment included 3 to 4 mice per group and was performed twice with similar results. H, Flow cytometry of Rae-1 expression on peritoneal macrophages of WT mice cultured in vitro in presence of oxidized low-density lipoprotein (OxLDL) and advanced glycation end products (AGE) as in C except that the dashed lines depict staining in the presence of a 10-fold excess of unlabeled anti-panRae-1 antibodies. The experiment was performed 3 times, with pools of cells from 3 mice for each, with similar results. I, Western blot analysis of Rae-1 proteins in macrophages cultured in vitro in presence of oxidized low-density lipoprotein or natural low-density lipoprotein (nLDL). The lipopolysaccharide (LPS) treatment was included as a positive control.33 The blot was performed 3 times with similar results. online-only Data Supplement), suggesting that these factors might not be directly associated with the MICA upregulation. We also performed immunohistochemical staining of atherosclerotic aortic sections of humans to assess expression of NKG2D ligands in the tissues. In contrast to plaque-free aortas (Figure 1J), atherosclerotic aortas contained many cells that stained positive with an anti-MICA/B antibody (Figure 1B, 1D, 1F, and 1H). The overlapping staining patterns of MICA/B with CD68 or CD31 in adjacent sections suggested that both macrophages and endothelial cells of the atheroscle- Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Xia et al NKG2D Promotes Atherosclerosis 2937 Figure 3. NKG2D plays a critical role in plaque formation in Western diet (WD)– fed and streptozotocin (STZ)-diabetic apolipoprotein E– deficient (ApoE⫺/⫺) mice. A, Visibly different plaque sizes in aortic arch regions of representative WD-fed ApoE⫺/⫺ and Klrk1⫺/⫺ApoE⫺/⫺ mice. The whitish plaque areas are indicated by arrows. The pictures shown are representative of 16 to 18 mice of each genotype. B, En face Oil red O staining to detect lipid-deposited plaque areas in aortas of representative ApoE⫺/⫺ (n⫽12) and Klrk1⫺/⫺ApoE⫺/⫺ (n⫽10) mice. Plaque areas stain red. The regions analyzed for percentage of plaque are boxed. C and D, Quantitative comparison of percentages of average Oil red O staining–positive plaque areas in the total aortic arch regions of Klrk1⫺/⫺ApoE⫺/⫺ and ApoE⫺/⫺ mice that were fed on a WD (C) or treated with streptozotocin (D). E, Quantitative comparison of percentages of average Oil red O staining–positive plaque areas in streptozotocin-induced diabetic ApoE⫺/⫺ mice treated with NKG2D or control antibodies (Ab). Total numbers of mice analyzed in C, D, and E are indicated in the graphs, which are compiled from data of 2 to 4 experiments for each model. rotic aortas expressed MICA/B (Figure 1B through 1I). The high expression of NKG2D ligands in aortic plaques suggested the potential involvement in atherosclerosis of immune activation mediated by NKG2D/ligand interactions. Preferential Upregulation of NKG2D Ligands in Tissue and Organs of ApoEⴚ/ⴚ Mice Where Abnormal Metabolites Accumulate To understand the NKG2D ligand upregulation process and its role in atherosclerosis, we used the ApoE⫺/⫺ mouse model of atherosclerosis. ApoE⫺/⫺ mice are defective in lipid metabolism and develop plaques when they age, particularly in susceptible regions such as aortic arch areas, and the disease is markedly exacerbated when the mice are fed the high-fat WD. We first assessed the amounts of transcripts for NKG2D ligands in RNA isolated from atherosclerotic aortic arch regions of WD-fed ApoE⫺/⫺ mice. Compared with those of either WD- or chow-fed wild-type (WT) C57BL/6 mice, samples from WD-fed ApoE⫺/⫺ mice had much higher (10 –30-fold) amounts of transcripts for Rae-1␦, Rae-1⑀, and H60b, which are mouse ligands for NKG2D (Figure 2A). These results suggest that the ligands are upregulated in the atherosclerotic aortas. Consistent with this conclusion, the atherosclerotic aortic arch regions of older chow-fed ApoE⫺/⫺ mice also had much higher amounts of transcripts for the NKG2D ligands than the atherosclerosis-resistant thoracic aortic region of the same mice (Figure II in the online-only Data Supplement). Immunohistochemical staining with Rae-1 and H60 antibodies confirmed the preferential expression of NKG2D ligands in plaques. Extensive Rae-1 and H60 staining was detected within plaques of WD-fed ApoE⫺/⫺ mice of the atherosclerotic aortic arch regions (Figure 2B, top and middle panels). In contrast, Rae-1 or H60 staining was nearly undetectable in plaque-free aortic regions of the same mice (Figure 2B, bottom). To determine the types of cells that express the ligands, we performed flow cytometric analysis of cells isolated from atherosclerotic aortas of WD-fed ApoE⫺/⫺ mice. In contrast to those of normal aortas, many macrophages and endothelial cells of the atherosclerotic aortas had appreciable cell surface Rae-1 expression (Figure 2C), consistent with the findings in humans. Because metabolic dysfunctions are systemic conditions, we tested whether other organs in the WD-fed ApoE⫺/⫺ mice had upregulated NKG2D ligands. Rae-1 transcripts were elevated dramatically in the liver and somewhat less so in the kidney, whereas other organs tested showed lower elevations (Figure 2D). Similar to the results with macrophages from aortic plaques, liver macrophages of ApoE⫺/⫺ mice contained significantly more Rae-1 transcripts than WT controls (Figure 2E), and cell surface expression of Rae-1 was also significantly, although modestly, increased (Figure 2F). Rae-1 was also markedly increased on the surface of hepatocytes of the ApoE⫺/⫺ mice, although WT hepatocytes exhibited a higher basal level of Rae-1 than did liver macrophages (Figure 2G). With the consideration that abnormal metabolites accumulate in both atherosclerotic plaques and livers, they may play a role in the induction of NKG2D ligands. Indeed, WT Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 2938 Circulation December 20/27, 2011 Figure 4. Preventing NKG2D/ligand interactions reduces serum levels of inflammation-associated cytokines in Western diet (WD)–fed and streptozotocin (STZ)-diabetic apolipoprotein E– deficient (ApoE⫺/⫺) mice. A, Serum levels of multiple cytokines in WD-fed ApoE⫺/⫺ and Klrk1⫺/⫺ApoE⫺/⫺ mice; n⫽12 each. B, Serum levels of multiple cytokines in streptozotocin-diabetic ApoE⫺/⫺ and Klrk1⫺/⫺ApoE⫺/⫺ mice; n⫽4 each. C, Serum levels of multiple cytokines of NKG2D antibody (Ab)–treated (n⫽9) and control antibody–treated (n⫽6) streptozotocin-diabetic ApoE⫺/⫺ mice. Note that scales for different cytokines in the different models are not the same. IL indicates interleukin; KC, Chemokine (C-X-C motif) ligand 1 (CXCL1); IFN, interferon; TNF, tumor necrosis factor; and BD, below detection limit. macrophages could be reproducibly induced to upregulate NKG2D ligands when cultured in vitro in the presence of oxidized LDL or advanced glycation end products, 2 abnormal metabolites associated with dyslipidemia and hyperglycemia (Figure 2H and 2I and Figure III in the online-only Data Supplement). The Rae-1 staining was competitively inhibited by unlabeled Rae-1 antibodies, confirming the specificity of the staining (Figure 2H). Together, these results suggest that abnormal metabolic conditions induce upregulation of NKG2D ligands in various tissues and organs, particularly those where abnormal metabolites accumulate, such as aortic plaques and livers. When it is considered that immune cell subsets such as NK and NKT cells in those tissues express NKG2D (Figure IV in the online-only Data Supplement) and promote atherosclerosis,6,9,11 the NKG2D/ ligand axis might play an important role in promoting atherosclerosis through activation of these or other NKG2Dexpressing cells. Preventing NKG2D/Ligand Interactions Suppresses Plaque Formation in ApoEⴚ/ⴚ Mice To directly determine the role of NKG2D in atherosclerosis, we tested whether interfering with NKG2D function suppresses plaque formation in ApoE⫺/⫺ mice. Compared with WD-fed ApoE⫺/⫺ mice, similarly fed NKG2D-deficient Klrk1⫺/⫺ApoE⫺/⫺ littermates exhibited dramatically smaller Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Xia et al NKG2D Promotes Atherosclerosis 2939 Table. Metabolic Parameters of NKG2D Knockout and Anti-NKG2D Antibody–Treated ApoEⴚ/ⴚ Mice in Western Diet and Streptozotocin Models of Atherosclerosis Western Diet ApoE⫺/⫺ (n⫽6) Streptozotocin-ApoE⫺/⫺⫹Antibody Streptozotocin ApoE⫺/⫺Klrk1⫺/⫺ (n⫽6) P ApoE⫺/⫺ (n⫽6) ApoE⫺/⫺Klrk1⫺/⫺ (n⫽6) P Control Antibody (n⫽5) NKG2D Antibody (n⫽4) P Cholesterol, mg/dL 706⫾89 337⫾48 0.005 1284⫾339‡ 1446⫾402 0.78 1537⫾528 1001⫾198 0.38 Triglycerides, mg/dL 98.3⫾7.6 57.5⫾6.8 0.002 85.0⫾8.0 57.5⫾13.5 0.22 84.8⫾16.3 81.5⫾19.7 0.9 Glucose, mg,dL 372⫾27* 308⫾14† 0.03 852⫾202§ 550⫾85 0.3 794⫾94 578⫾53 0.1 *Fourteen samples were analyzed. †Twelve samples were analyzed. ‡P⬍0.05 compared with the cholesterol concentrations of Western diet–fed apolipoprotein E– deficient (ApoE⫺/⫺) mice. §P⬍0.001 compared with the glucose concentrations of Western diet–fed ApoE⫺/⫺ mice. plaques in the aortas (Figure 3A through 3C). On average, the percentage of lipid-deposited plaque area in the total aortic arch region, as assessed by en face Oil red O staining, was reduced 5- to 6-fold in Klrk1⫺/⫺ApoE⫺/⫺ mice (Figure 3B and 3C). Oil red O staining of cross sections of aortic roots also revealed significantly reduced plaque formation in Klrk1⫺/⫺ApoE⫺/⫺ mice compared with the ApoE⫺/⫺ controls, but the effect was less dramatic than seen in the aortic arch regions (Figure VA in the online-only Data Supplement). The different effects of NKG2D knockout on the plaque reduction in the aortic arch region and aortic roots are consistent with other reports showing that immune-mediated mechanisms differentially influence plaque formation in different anatomic sites.36,37 To test whether the NKG2D/ligand axis is broadly involved in atherosclerosis that accompanies diabetic conditions, we also examined streptozotocin-treated ApoE⫺/⫺ mice. Because it is directly toxic for insulin-secreting pancreatic -cells, streptozotocin causes severe hyperglycemia and hyperlipidemia in mice by a mechanism similar to that of type 1 diabetes mellitus.27 As in the WD-fed model, streptozotocin-treated Klrk1⫺/⫺ApoE⫺/⫺ mice had much smaller plaque sizes than similarly treated ApoE⫺/⫺ mice in aortic arch regions (Figure 3D). The NKG2D knockout also significantly, although less dramatically, reduced the amount of plaque in the aortic root cross sections in the streptozotocin-diabetic ApoE⫺/⫺ mice (Figure VB in the online-only Data Supplement). As an additional approach to test the role of NKG2D in atherosclerosis, which might be also relevant for therapeutic applications, we tested whether the induction of atherosclerosis in streptozotocin-diabetic ApoE⫺/⫺ mice was inhibited by injection of NKG2D antibodies that are known to block interactions of NKG2D with its ligands.16,24 Compared with ApoE⫺/⫺ mice injected with control antibodies, those that received the NKG2D antibodies had markedly reduced plaque areas (Figure 3E). Together, these results demonstrate a critical role of the NKG2D/ligand axis in atherosclerosis induced under various abnormal metabolic conditions. Preventing NKG2D/Ligand Interactions Reduces the Systemic Production of Multiple Cytokine Markers Associated With Immune Activation Because the NKG2D/ligand interaction activates NKG2Dexpressing immune cells, which produce inflammatory cyto- kines, preventing the interaction likely suppresses atherosclerosis by inhibiting immune activation. In support of this conclusion, compared with ApoE ⫺/⫺ mice, NKG2Dknockout or NKG2D antibody–treated ApoE⫺/⫺ mice exhibited significant reductions in the amounts of multiple cytokines in the serum, including inflammatory cytokines (IL-6, IFN-␥) and an immune regulatory cytokine (IL-10) (Figure 4). IL-6 and IFN-␥ are known to be involved in atherosclerosis38 and were markedly reduced in all of the models in which NKG2D function was prevented, suggesting that NKG2D/ligand-mediated immune cell activation associated with atherosclerotic progression might function at least partly through the increased production of these cytokines. IL-12 was also reduced in all of the models, although the difference was not significant in the streptozotocin model. The other cytokines studied showed more variable results. NKG2D-Mediated Immune Activation Aggravates Abnormal Metabolic Conditions by Promoting Liver Inflammation and Dysfunction Preventing NKG2D interactions also considerably alleviated the abnormal metabolic conditions in the WD-fed ApoE⫺/⫺ model. Notably, the sera of WD-fed Klrk1⫺/⫺ApoE⫺/⫺ mice were much less cloudy than the sera of similarly fed ApoE⫺/⫺ mice (Figure VI in the online-only Data Supplement), suggesting reduced lipid content. Direct analysis showed that cholesterol and triglycerides were significantly reduced in the Klrk1⫺/⫺ApoE⫺/⫺ mice (Table). The glucose levels were also significantly, although modestly, reduced in the WD-fed Klrk1⫺/⫺ApoE⫺/⫺ mice (Table). Compared with WD-fed ApoE⫺/⫺ mice, streptozotocintreated ApoE⫺/⫺ mice had more severe hyperglycemia and hyperlipidemia, neither of which were significantly corrected by interfering with NKG2D (Table). These findings suggest that the severe metabolic dysfunctions in the streptozotocin model are not dependent on NKG2D-mediated immune activation, consistent with the notion that streptozotocin induces diabetes mellitus through its direct toxic effect on pancreatic -cells and other cells.39 Nevertheless, interfering with NKG2D in the streptozotocin model suppressed production of the cytokines associated with immune activation and atherosclerosis (Figure 3D), suggesting that preventing NKG2D-mediated inflammation reduces atherosclerosis without the requirements of alleviating overall metabolic dysfunctions. However, in the WD model, the role of Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 2940 Circulation December 20/27, 2011 ligands in the ApoE⫺/⫺ mice, we tested whether the NKG2D/ ligand axis contributes to liver inflammation that results in liver dysfunction. Compared with WD-fed ApoE⫺/⫺ mice, WD-fed Klrk1⫺/⫺ApoE⫺/⫺ mice exhibited significantly lower levels of serum alanine aminotransferase activities (Figure 5A), indicating that their liver dysfunction was alleviated. Furthermore, liver explants from Klrk1⫺/⫺ApoE⫺/⫺ mice, cultured ex vivo, produced significantly less IL-6 than those from ApoE⫺/⫺ mice, indicative of reduced inflammation (Figure 5B). In addition, the numbers of macrophages, NKT cells, and NK cells were also significantly lower in livers of Klrk1⫺/⫺ApoE⫺/⫺ mice than ApoE⫺/⫺ mice (Figure 5C). These data suggest that NKG2D-mediated immune activation is directly involved in liver inflammation and dysfunction, which is known to promote abnormal metabolic conditions. NKG2D/Ligand Interaction Implicates Multiple Immune Cell Populations in Liver Inflammation To gain insight into cellular mechanisms that underlie NKG2D-mediated liver inflammation, we determined the influence of NKG2D deficiency on activation of different immune cell populations in the WD-fed ApoE⫺/⫺ mice. The cell types most likely to be affected are those that express NKG2D, including NK and NKT cells in the liver (Figure IV in the online-only Data Supplement). Indeed, NKT cells and, to a lesser extent, NK cells from livers of the ApoE⫺/⫺ mice exhibited considerable production of IFN-␥, which was markedly attenuated when the mice lacked NKG2D (Figure 6A and 6B). In contrast, few conventional ␣ T cells of the liver expressed IFN-␥ (not shown), and the liver macrophages produced high levels of IL-6 in ApoE⫺/⫺ mice whether or not the mice expressed NKG2D (Figure 6C). These results suggest that the production of IL-6 by macrophages is independent of NKG2D, consistent with the fact that they did not express NKG2D (Figure IV in the online-only Data Supplement). However, because liver macrophages were markedly reduced in Klrk1⫺/⫺ApoE⫺/⫺ mice and they expressed NKG2D ligands in atherogenic conditions (Figures 2E and 2F and 5C), these cells likely stimulate NKG2Dmediated liver inflammation and at the same time are influenced by it. Figure 5. Preventing NKG2D/ligand interactions alleviates abnormal metabolic conditions by reducing liver inflammation in the Western diet–fed apolipoprotein E– deficient (ApoE⫺/⫺) model of atherosclerosis. A, Reduced serum alanine aminotransferase (ALT) activity in Western diet–fed Klrk1⫺/⫺ApoE⫺/⫺ mice. The experiment included 5 mice per group and was representative of 2 repeated experiments of a total of 10 mice. B, Reduced interleukin (IL)-6 production by ex vivo cultured liver explants of Klrk1⫺/⫺ApoE⫺/⫺ mice. Experiment includes 3 mice per assay and was representative of 2 repeated experiments of a total of 6 mice. C, Reduced numbers of immune cells in livers of Klrk1⫺/⫺ApoE⫺/⫺ mice. The numbers are of each type of immune cells per liver, calculated on the basis of total mononucleocytes isolated from livers and percentages of each type. The experiment included 3 mice per assay and was representative of 3 repeated experiments of a total of 9 mice. WT indicates wild-type. NKG2D in aggravating metabolic conditions may also contribute to the atherosclerosis. Because the liver is the major organ of lipid metabolism and exhibited highly upregulated expression of NKG2D Discussion The studies presented here identify the NKG2D/ligand axis as a molecular link between chronic inflammation and metabolic dysfunction that is critical for atherosclerosis and contributes to type 2 diabetes mellitus progression. When it is considered that the NKG2D ligands were upregulated in multiple tissues, it can be seen that NKG2D-mediated immune activation likely promotes atherosclerosis through several mechanisms. In aortas, preferential upregulation of the NKG2D ligands is associated with enhanced plaque formation in arch regions, suggesting that it is directly involved in atherosclerotic progression. NKG2D/ligand-mediated immune activation could enhance the inflammation in atherosclerotic aortas, which in turn might affect recruitment, survival, and function of ligand-expressing macrophages that are directly involved in plaque progression.40 Although upregulation of NKG2D Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Xia et al NKG2D Promotes Atherosclerosis 2941 Figure 6. Multiple immune cell types are involved in the liver inflammation mediated by NKG2D interactions. A, Comparison of the production of interferon (IFN)-␥ by liver NKT cells of Western diet–fed Klrk1⫺/⫺apolipoprotein E– deficient (ApoE⫺/⫺) and ApoE⫺/⫺ mice. Cells are gated on TCR⫹NK1.1⫹ cells. B, Comparison of IFN-␥ production by liver natural killer (NK) cells of Western diet– fed Klrk1⫺/⫺ApoE⫺/⫺ and ApoE⫺/⫺ mice. Cells are gated on CD3⫺NK1.1⫹ cells. C, On a per cell basis, NKG2D deficiency did not affect interleukin (IL)-6 production by liver CD11b⫹F4/80⫹ macrophages of Western diet–fed ApoE⫺/⫺ mice. The experiment in A through C included 3 mice per group and is representative of 3 repeated experiments of a total of 9 mice. The number below the gate in each graph is the mean⫾SE percentage of the positive cells of total cells in the plot. The isotype-control staining was similar in wild-type (WT), ApoE⫺/⫺, and Klrk1⫺/⫺ApoE⫺/⫺ mice. ***, *Statistical significance compared with values from ApoE⫺/⫺ mice. ligands is most profound in developed plaques, we found that enhanced expression of the ligands was apparent in aortas of ApoE⫺/⫺ mice as early as 8 weeks of age, before plaques were detected (data not shown), suggesting that the NKG2D/ligand axis might be involved in early stages of atherosclerosis. NKG2D/ligand-mediated immune activation could also promote atherosclerosis by exacerbating metabolic dysfunctions and causing systemic inflammation in the liver and potentially other tissues. In this regard, the abnormal upregulation of NKG2D ligands in the pancreas of mice with nonobese diabetes, an autoimmune type 1 diabetes mellitus model, reportedly contributes to immune destruction of -cells and development of diabetes mellitus.24 Although the underlying mechanisms are different, NKG2D also plays a role in progression of type 2 diabetes mellitus in the studies presented here. Therefore, the NKG2D/ligand axis is extensively involved in multiple aspects of diabetes mellitus and atherosclerosis. It will be interesting to determine whether the NKG2D/ligand axis is involved in the development of other diabetes mellitus–associated complications, such as retinopathy, nephropathy, and neuropathy. It is likely that NKG2D exerts its disease-causing effects in multiple cell types. We observed a marked reduction in NK and NKT cell activation in livers of WD-fed ApoE⫺/⫺ mice, suggesting that NKG2D/ligand interactions may be necessary for activating these cells and that such activation may promote metabolic dysfunctions and atherosclerosis. Because of the very limited numbers of cells, we could not perform a similar analysis of cells isolated from atherosclerotic plaques. Both NK and NKT cells have been reported to contribute to the severity of atherosclerosis in similar mouse models. Although it is difficult to compare different studies directly, the published studies suggest that the contribution of NK or NKT cells individually to atherosclerosis is less marked than the contribution of NKG2D shown here. Therefore, it is possible that both populations, along with other NKG2D⫹ cells such as ␥␦T cells, contribute to the disease in an NKG2D-dependent fashion and that NKG2D deficiency therefore has a greater effect on the disease than either of the NKG2D⫹ cell populations do separately. We also found that the NKG2D ligands are upregulated in blood and plaques of patients with type 2 diabetes mellitus, suggesting the potential clinical application of blocking NKG2D/ligands in treatment of atherosclerosis or metabolic disease in patients. However, additional preclinical studies are necessary before this approach can be considered for clinical use. It remains to be determined whether blockade of NKG2D affects established plaques, for example. Another issue that needs further study is whether binding of NKG2D antibody to NKG2D induces global inhibition of the functions of NKG2D-expressing cells, which was suggested by some studies41 but refuted by others.42 Although we cannot rule out that this occurs in our antibody blockade studies, the NKG2D knockout experiments provide a direct and compelling line of evidence that NKG2D is involved in the disease. In conclusion, our findings provide new insights into the mechanisms underlying association of inflammation with atherosclerosis and type 2 diabetes mellitus and suggest possible new approaches to therapy of these diseases. In addition, the observation that sMICA is elevated in sera of diabetic patients suggests that sMICA measurement may have utility as a biomarker of atherosclerotic progression. Acknowledgments Dr Xia performed all of the experiments except the immunohistochemical staining. Drs Shi and Sukhova performed the immunohistochemical staining of human and mouse tissues. Dr Yang was involved in the immunohistochemical staining of mouse tissues. Dr Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 2942 Circulation December 20/27, 2011 Miller was involved in analysis of blood of patients with diabetes mellitus. Drs Raulet and Guerra generated NKG2D antibodies and NKG2D knockout mice. Dr Xiong initiated the project, and Drs Xiong, Xia, and Raulet designed the experiments, analyzed data, and prepared the manuscript. Sources of Funding This study was supported by an institutional fund of Pennsylvania State University, a fund from the Penn State Institute of Diabetes and Obesity (to Dr Xiong), and grants from the National Institutes of Health (to Dr Raulet). Disclosures Dr Raulet received grant support from Novo Nordisk. Drs Xiong, Xia, and Raulet received payments from Novo Nordisk through Pennsylvania State University and University of California, Berkeley. Drs Guerra, Yang, Shi, Sukhova, and Miller report no conflicts. References 1. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399 – 409. 2. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. 3. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860 – 867. 4. Kleindienst R, Xu Q, Willeit J, Waldenberger FR, Weimann S, Wick G. Immunology of atherosclerosis: demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol. 1993;142: 1927–1937. 5. Bobryshev YV, Lord RS. Identification of natural killer cells in human atherosclerotic plaque. Atherosclerosis. 2005;180:423– 427. 6. Whitman SC, Ramsamy TA. Participatory role of natural killer and natural killer T cells in atherosclerosis: lessons learned from in vivo mouse studies. Can J Physiol Pharmacol. 2006;84:67–75. 7. Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1997;94:4642– 4646. 8. Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of CD4⫹ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864 – 870. 9. Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199:417– 422. 10. Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178 –180. 11. Whitman SC, Rateri DL, Szilvassy SJ, Yokoyama W, Daugherty A. Depletion of natural killer cell function decreases atherosclerosis in lowdensity lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol. 2004;24:1049 –1054. 12. Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679 –10684. 13. Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149 –3156. 14. Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelialdependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234 –247. 15. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. 16. Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19 –29. 17. Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A. 1996; 93:12445–12450. 18. Cerwenka A, Bakker ABH, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. 19. Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119 –126. 20. Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186 –1190. 21. Lodoen M, Ogasawara K, Hamerman JA, Arase H, Houchins JP, Mocarski ES, Lanier LL. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197:1245–1253. 22. Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100: 9452–9457. 23. Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, Ebert EC, Green PH, Jabri B. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. 24. Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 2004;20:757–767. 25. Hue S, Mention JJ, Monteiro RC, Zhang S, Cellier C, Schmitz J, Verkarre V, Fodil N, Bahram S, Cerf-Bensussan N, Caillat-Zucman S. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–377. 26. Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. 27. Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4: 1025–1031. 28. Calkin AC, Forbes JM, Smith CM, Lassila M, Cooper ME, Jandeleit-Dahm KA, Allen TJ. Rosiglitazone attenuates atherosclerosis in a model of insulin insufficiency independent of its metabolic effects. Arterioscler Thromb Vasc Biol. 2005;25:1903–1909. 29. Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940 –945. 30. Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. 31. Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2Dligand interaction and natural killer cells. Hepatology. 2007;46:706 –715. 32. Takada A, Yoshida S, Kajikawa M, Miyatake Y, Tomaru U, Sakai M, Chiba H, Maenaka K, Kohda D, Fugo K, Kasahara M. Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. J Immunol. 2008;180: 1678 –1685. 33. Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol. 2004;172:2001–2005. 34. Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169: 4098 – 4102. 35. Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419: 734 –738. 36. Peng D, Hiipakka RA, Reardon CA, Getz GS, Liao S. Differential anti-atherosclerotic effects in the innominate artery and aortic sinus by the liver X receptor agonist T0901317. Atherosclerosis. 2009;203:59 – 66. 37. Reardon CA, Blachowicz L, Gupta G, Lukens J, Nissenbaum M, Getz GS. Site-specific influence of polyunsaturated fatty acids on atherosclerosis in immune incompetent LDL receptor deficient mice. Atherosclerosis. 2006; 187:325–331. Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Xia et al 38. Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. 39. Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007;191:241–249. 40. Tabas I, Seimon T, Timmins J, Li G, Lim W. Macrophage apoptosis in advanced atherosclerosis. Ann N Y Acad Sci. 2009;1173(suppl 1): E40 –E45. NKG2D Promotes Atherosclerosis 2943 41. Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–3578. 42. Champsaur M, Beilke JN, Ogasawara K, Koszinowski UH, Jonjic S, Lanier LL. Intact NKG2D-independent function of NK cells chronically stimulated with the NKG2D ligand Rae-1. J Immunol. 2010;185: 157–165. CLINICAL PERSPECTIVE It is well established that immune activation is involved in progression of atherosclerosis in patients with diabetes mellitus. However, molecular events stimulating the immune activation under diabetic conditions are not well understood, hindering our effort to modulate the immune system for prevention and/or treatment of the disease. We report herein that a group of stimulating ligands for the immune-activating receptor NKG2D were upregulated in tissues such as aorta and liver in diabetic mice or patients and that inhibiting the NKG2D/ligand interaction suppressed atherosclerosis and metabolic dysfunction by preventing NKG2D-expressing immune cell–mediated tissue inflammation in model mice. These findings suggest the potential application of targeting NKG2D/ligands in prevention/treatment of atherosclerosis and abnormal metabolic conditions in diabetic patients. Because expression of the NKG2D ligands could be upregulated in other affected tissues, NKG2D/ligands might be also involved in other diabetes mellitus–associated complications, such as retinopathy and nephropathy. Furthermore, our finding that preventing the NKG2D/ligand-mediated inflammation could suppress atherosclerosis even under constantly severe abnormal metabolic conditions suggests that immune activation contributes to atherosclerosis through separate, although overlapping, processes than metabolic dysfunction does. Therefore, combined treatment of both metabolic dysfunction and immune activation might represent a better therapeutic strategy than the treatment of either symptom alone. Finally, our observation that levels of soluble NKG2D ligands were elevated differentially in blood of patients with type 2 diabetic mellitus suggests its potential utility as a biomarker in predicting progression of atherosclerosis and other complications. Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 SUPPLEMENTARY MATARIAL Supplementary Table 1. Clinical characteristics of sMICA-positive vs. sMICA-negative type 2 diabetic patients. ID# MICA+ patients P values sMICA (pg/ml) Age (Yrs) Years with diabetes 1 2 12250 2100 41 66 1 10 158 159 3 4 598 533 59 59 4 9 264 157 5 6 7 440 431 350 62 63 47 1 1 1.5 124 279 94 8 9 156 91 67 60 4 1 134 158 10 ‡ Avg ±SD 39 1698 ±3753 68 59.2 ±8.7 0.63 3 3.6 ±3.4 0.29 131 165.8 ±59.5 0.61 57.3 ±9.2 58 61 62 6.1 ±5.3 4 2 3 157.2 ±47.2 101 206 132 56 41 2 5 151 201 68 2 113 62 10 125 60 2 154 ‡ Avg ±SD 11 12 13 14 15 MICApatients † BD BD BD BD BD BD BD 16 Blood glucose (mg/dL) BD 17 BD 18 BD 19 20 21 BD BD 41 68 63 10 20 5 129 118 205 22 BD 48 4 251 Medical Condition high BP* heart disease High BP, Osteoporosis gall bladder Kidney Stones none noted high cholesterol high BP, high cholesterol none noted high cholesterol, high BP high cholesterol high BP Stents, high BP high cholesterol Heart palpatations high cholesterol, high BP high cholesterol, high BP high BP, high cholesterol high BP, high cholesterol high cholesterol high BP high BP, high cholesterol Medication Zocor (simvastatin) none noted Zocor (simvastatin) Zocor (simvastatin) Vytorin (simvastatin, ezetimibe) none noted none noted Zetia (ezetimibe) simvastatin Lovastat Zetia, Avandia none noted Zetia (ezetimibe) Lipitor (atorvastatin) none noted none noted Lipitor (atorvastatin) none noted none noted Zocor (simvastatin) none noted Zocor (simvastatin) Note: Ages of the healthy controls (N=19) are 52.1±2.5 years and no medications are noted for them. *high BP: high blood pressure; †BD: below the detection limit; ‡ Avg±SD: Average± standard deviation. Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Supplementary Figure 1. Genotyping of Klrk1-/-ApoE-/- mice. For genotyping of ApoE knockouts, genomic DNA was PCR amplified with a set of three primers that produced a 245-bp band of the ApoE knockout allele and a 155-bp band of the wild type ApoE allele in one PCR reaction. Sequences of the three primers used for the ApoE knockout genotyping were 0IMR0180:GCC TAG CCG AGG GAG AGC CG; 0IMR0181: TGT GAC TTG GGA GCT CTG CAG C; and 0IMR0182: GCC GCC CCG ACT GCA TCT (The Jackson laboratory). For genotyping of Klrk1 knockouts, genomic DNA was PCR amplified with a set of three primers that yielded a 422-bp band for the wild-type Klrk1 allele and a 317-bp band for the Klrk1 knockout allele in one PCR reaction. The three primers used for the Klrk1 genotyping were published28. WT: wild type. KO: knockout. 2 Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Supplementary Figure 2. Real-time RT-PCR analysis of transcripts for the NKG2D ligands Rae-1δ, Rae-1ε and H60b in arch and thoracic regions of aortae of same 8 month-old ApoE-/mice feeding on normal chow. The results are presented as ratios of the expression levels in the arch vs. thoracic regions of aortae. The expression levels of the NKG2D ligands are normalized on the β-actin levels. **P<0.01 and ***P<0.001 are calculated based on comparison of the expression levels of the individual NKG2D ligands in the arch vs. thoracic regions of aortae, averaged of three mice. 3 Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Supplementary Figure 3. Real-time RT-PCR analysis for transcripts of different NKG2D ligands in wild-type macrophages cultured in vitro in presence of oxLDL or nLDL. The culture condition of the macrophages was described in the Method section. The results are of three assays. **P<0.01, ***P<0.001. 4 Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Supplementary Figure 4. The NKG2D expression on various subsets of immune cells isolated from livers, spleens and aortae of WD-fed ApoE-/- or normal wild type mice. Mononucleocytes isolated from livers or aortae or splenocytes were immunofluorescently stained and analyzed by flow cytometry for NKG2D expression on gated macrophages (CD11b+F4/80+), NKT (CD3+NK1.1+), NK (CD3-NK1.1+) and conventional αβT cells (CD3+NK1.1-TCRβ+). The gray areas are of staining with isotype control antibodies. In the samples of livers, the cells of NKG2D-knockout Klrk1-/-ApoE-/- mice are included as additional negative controls for the NKG2D staining. The experiment was done twice. Few NK and NKT cells could be isolated from aortae of normal wild type mice for the reliable analysis and not shown. 5 Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Supplementary Figure 5. Oil red O staining to detect lipid-deposition in aortic roots of WD-fed (A) or STZ-induced diabetic (B) ApoE-/- and Klrk1-/-ApoE-/- mice. Plaque areas stained red. Representatives of the staining of each type were shown in the left and middle panels. Quantitative comparison of Oil red O staining positive areas of each type was shown in the right panels, compiled from results of four to five mice for each type. 6 Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013 Supplementary Figure 6. Clearer appearance of sera of WD-fed Klrk1-/-ApoE-/- mice than of similarly fed ApoE-/- mice. 7 Downloaded from http://circ.ahajournals.org/ at UNIV OF CALIFORNIA LIB on January 29, 2013