* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Recognition of bacteria by inflammasomes.
Survey
Document related concepts
Adoptive cell transfer wikipedia , lookup
Major urinary proteins wikipedia , lookup
Adaptive immune system wikipedia , lookup
Complement system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immune system wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Molecular mimicry wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Transcript
IY31CH04-Vance ARI 24 February 2013 ANNUAL REVIEWS Further 11:58 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. Click here for quick links to Annual Reviews content online, including: • Other articles in this volume • Top cited articles • Top downloaded articles • Our comprehensive search Recognition of Bacteria by Inflammasomes Jakob von Moltke, Janelle S. Ayres, Eric M. Kofoed, Joseph Chavarrı́a-Smith, and Russell E. Vance Department of Molecular & Cell Biology, Division of Immunology and Pathogenesis, University of California, Berkeley, California 94720; email: [email protected] Annu. Rev. Immunol. 2013. 31:73–106 Keywords First published online as a Review in Advance on November 26, 2012 caspase, NLR, innate immunity, pyroptosis The Annual Review of Immunology is online at immunol.annualreviews.org Abstract This article’s doi: 10.1146/annurev-immunol-032712-095944 c 2013 by Annual Reviews. Copyright ! All rights reserved Inflammasomes are cytosolic multiprotein complexes that assemble in response to a variety of infectious and noxious insults. Inflammasomes play a critical role in the initiation of innate immune responses, primarily by serving as platforms for the activation of inflammatory caspase proteases. One such caspase, CASPASE-1 (CASP1), initiates innate immune responses by cleaving pro-IL1β and pro-IL-18, leading to their activation and release. CASP1 and another inflammatory caspase termed CASP11 can also initiate a rapid and inflammatory form of cell death termed pyroptosis. Several distinct inflammasomes have been described, each of which contains a unique sensor protein of the NLR (nucleotidebinding domain, leucine-rich repeat–containing) superfamily or the PYHIN (PYRIN and HIN-200 domain–containing) superfamily. Here we describe the surprisingly diverse mechanisms by which NLR/PYHIN proteins sense bacteria and initiate innate immune responses. We conclude that inflammasomes represent a highly adaptable scaffold ideally suited for detecting and initiating rapid innate responses to diverse and rapidly evolving bacteria. 73 IY31CH04-Vance ARI 24 February 2013 11:58 INTRODUCTION Caspase: cysteine aspartic acid–specific protease NBD: nucleotide-binding domain Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. LRR: leucine-rich repeat NLR: nucleotidebinding domain, leucine-rich repeat– containing TLR: Toll-like receptor CARD: caspase activation and recruitment domain ASC: apoptosisassociated speck-like protein containing a CARD PYHIN: PYRIN and HIN-200 domain– containing Autoinhibited NBD-LRR NBD PYD NBD PYD The term inflammasome was first coined by Tschopp and colleagues (1) in 2002 to describe a high-molecular-weight protein complex that forms in the cytosol and serves as a platform for the recruitment and autoproteolytic activation of certain caspases, notably CASP1, CASP4, and CASP5 (Figure 1). These caspases, along with CASP11 and CASP12 in the mouse, are termed the inflammatory caspases and are distinct from the caspases involved in the induction of apoptosis. CASP1 is the best characterized of the inflammatory caspases and was first described as the protease required for the proteolytic cleavage of pro-IL-1β and pro-IL-18, resulting in their functional maturation and release (2, 3). In the decade since the initial description of an inflammasome, it has become clear that there are in fact multiple distinct inflammasomes, each of which is activated by unique stimuli that can include infectious agents as well as noninfectious stimuli. The formation of each inflammasome is dictated by a unique scaffolding protein (Figure 2). Most of these scaffolding proteins contain a nucleotide-binding domain (NBD) and leucine-rich repeats (LRRs) and are thus members of the NBD-LRR (NLR) superfamily. The LRRs are believed to have two functions (Figure 1). First, LRRs are thought to be Monomeric CASP1 Ligand CARD p10 required to maintain NLRs in an autoinhibited state because deletion of LRRs generally leads to a constitutively active NLR. Second, by analogy to the well-characterized ligand binding function of LRR domains in Toll-like receptors (TLRs), the LRRs of NLRs are believed to mediate recognition of pathogenderived (or potentially self-derived) ligands. As discussed below, however, evidence for ligand recognition by NLRs is weak. The NBD is believed to mediate the assembly of NLRs into an oligomerized state that is critical for the induction of downstream signaling. In NLRs, signaling is initiated by CARDs (caspase activation and recruitment domains) that can recruit CASP1 directly or by PYRIN domains that recruit CASP1 via the CARD-PYRINcontaining adaptor protein ASC (Figure 2). Once recruited to an oligomerized inflammasome, CASP1 is activated via dimerization and autoproteolysis (Figure 1). Interestingly, non-NLR proteins such as PYRIN-HIN200 domain–containing (PYHIN) proteins have also been shown to mediate CASP1 activation (4–8). Although PYHIN proteins lack NBDs and LRRs, they may nevertheless exhibit the properties of autoinhibition and ligand-induced oligomerization (9). It has become clear that inflammasomes not only have diverse mechanisms of activation but can also have diverse functions that extend beyond cytokine processing. Most notably, Inflammasome complex Pyroptosis p20 CASP1 autoprocessing ATP Oligomerized NBD-LRR PYD CARD ASC (adaptor) Pyroptosis IL-1β/IL-18 Other? Figure 1 Generic model of inflammasome activation. Cytosolic nucleotide-binding domain, leucine-rich repeat (NBD-LRR) proteins are maintained in an autoinhibited state until ligand recognition and ATP binding drive oligomerization via the NBD domain. Recruitment of CASP1 to form the inflammasome complex occurs directly via CARD-CARD interactions or indirectly via the adaptor ASC. Monomeric CASP1 is believed to be activated by proximity-induced autoproteolysis (autoprocessing), leading to downstream effector functions such as pyroptosis and processing of pro-IL-1β and pro-IL-18. In some cases, unprocessed CASP1 can also initiate pyroptosis. Similar mechanisms are postulated for the other inflammatory caspases (CASP4, CASP5, CASP11, and CASP12) but have not been well characterized. Abbreviations: CARD, caspase activation and recruitment domain; PYD, PYRIN domain. 74 von Moltke et al. Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 inflammasome activation is frequently associated with a rapid and lytic form of host cell suicide called pyroptosis (10). Inflammasome activation plays a critical role in mediating host defense, but inappropriate or excessive inflammasome activation can also be detrimental to host health. Inflammasomes are therefore a complex and surprisingly diverse set of cytosolic sensors that must be stringently regulated. Here we focus on inflammasome activation by bacteria. We survey what is known about how bacteria activate inflammasomes and ask whether it is possible to discern any general principles that govern inflammasome activation or function in host defense. The discovery of NLRs in mammals was predated by work demonstrating that innate immunity in plants depends largely on diverse NBD-LRR proteins (reviewed in 11, 12). Just as inflammasomes induce a pyroptosis response, plant NBD-LRR proteins often induce a rapid cell death response, termed the hypersensitive response, that is believed to restrict pathogen replication at sites of infection. Despite the strikingly similar domain architectures and functions of mammalian and plant NBD-LRR proteins, evolutionary analyses strongly suggest that NBD-LRR proteins were absent in the common ancestor of plants −−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−→ Figure 2 Murine inflammasome proteins and their domains. NBD-LRR family members can be categorized according to the unique domains they encode: ! NLRP1: FIIND; " all other NLRP proteins: PYRIN domain (PYD); # NLRC: CARD; $ NAIP: BIR domains. % AIM2 belongs to the PYHIN family of proteins and uses the HIN-200 domain for ligand recognition. & NLRP10 is the only family member lacking an LRR domain. ' ASC is an adaptor required by NLRP proteins for recruitment of CASP1. ( CASP1 contains a CARD and a protease domain that autoprocesses into p10 and p20 subunits. CASP11 and CASP12 are additional inflammatory caspases in mice; CASP4 and CASP5 are additional inflammatory caspases in humans. Other important differences between murine and human proteins are listed (below). (See text for abbreviations.) and animals and thus evolved independently (convergently) in each lineage (13, 14). The independent evolution of plant and animal NBD-LRR proteins raises a key question: What about the conjunction of an NBD and an LRR is so fundamentally advantageous that evolution would strike at least twice upon this protein architecture as a solution to the problem of effective innate immune defense? NLRP1A-C 1 NBD FIIND CARD LRR NLRP2, 3, 4A-G, 5, 6, 9A-C, 12, 14 2 PYD NBD LRR NLRC1-5* 3 LRR CARD NBD * NLRC1 = NOD1; NLRC2 = NOD2 and has 2× CARD NAIP1, 2, 5, 6 4 BIR BIR BIR NBD LRR AIM2 5 PYD HIN-200 NLRP10 6 PYD NBD ASC 7 PYD CARD CASP1 8 CARD p10 p20 Humans versus mice: • Humans express only one NLRP1 and it has • both a PYD and a CARD • Humans express only one NAIP • Humans express NLRP7, 8, 11, and 13, which • are absent in mice • Humans express only one NLRP4 www.annualreviews.org • Recognition of Bacteria by Inflammasomes 75 IY31CH04-Vance ARI 24 February 2013 NLRC: NLR family, CARD-containing T3SS: bacterial type III secretion system T4SS: bacterial type IV secretion system Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. NAIP: NLR family, apoptosis inhibitory protein (formerly known as BIRC) 76 11:58 By articulating the key principles underlying inflammasome activation by bacteria, this review attempts to address this question. RECOGNITION OF BACTERIA BY INFLAMMASOMES The NAIP/NLRC4 Inflammasome Activation of NLRC4. NLRC4 (also known as IPAF, CLAN, and CARD12) was identified on the basis of similarity to apoptotic protease-activating factor-1 (APAF-1) and was shown to recruit and activate CASP1 (15–17). Initial studies of Nlrc4−/− mice demonstrated that Nlrc4 is essential for CASP1 activation by Salmonella enterica serovar Typhimurium (S. Typhimurium) (18). Since then, CASP1 activation by a large number of additional pathogens, including Legionella pneumophila (19–21), Pseudomonas aeruginosa (22–24), and Shigella flexneri (25), has been shown to depend on NLRC4. Activation of the NLRC4 inflammasome by bacteria depends largely [e.g., S. Typhimurium (26–28)] or almost entirely [e.g., L. pneumophila (19, 20, 27, 29)] on bacterial flagellin expression. Indeed, the cytosolic presence of the flagellin protein—or just its C-terminal 35 amino acids—is sufficient to activate NLRC4 (26–29). C-terminal truncation of the flagellin protein (20) or mutation of three conserved C-terminal hydrophobic amino acids (27) abolished recognition of flagellin by NLRC4 (20, 26, 27, 30–33). The C terminus comprises part of the D0 region of flagellin and is distinct from the D1 region, which is recognized by the TLR5 cell-surface flagellin receptor (34, 35). Importantly, direct binding of flagellin to NLRC4 has not been demonstrated, and in fact, flagellin activation of NLRC4 appears to be indirect (see below). Activation of NLRC4 by flagellated bacteria also requires bacterial expression of specialized secretion systems, such as the S. Typhimurium SPI-1 type III secretion system (T3SS) or the L. pneumophila Dot/Icm type IV secretion system (T4SS). The physiological role of these secretion systems is to translocate bacterial virulence von Moltke et al. factors into the host cell cytosol, but considerable evidence suggests they can also translocate flagellin (27, 36). Cytosolic translocation of flagellin is presumably inadvertent, because flagellin has no known function in the host cell cytosol. The natural hosts of L. pneumophila are amoebae, not mammals. Thus L. pneumophila may not have experienced selective pressure to evade NLRC4, whereas other mammalianadapted pathogens (e.g., S. Typhimurium and Listeria monocytogenes) appear to have evolved regulatory mechanisms that limit flagellin expression in host cells (see below). Interestingly, several flagellin-deficient bacteria, most notably Shigella, are also capable of activating NLRC4 (22, 25, 31, 32, 37). Activation of NLRC4 by these pathogens requires expression of a T3SS (22, 25, 31, 32, 37). Miao and colleagues (31) provided an explanation for the flagellin-independent activation of NLRC4 by demonstrating that the inner rod proteins of several bacterial T3SSs are capable of activating NLRC4. Like the D0 domain of flagellin that activates NLRC4, the T3SS inner rod proteins are believed to adopt an α-helical secondary structure and oligomerize to form a proteintranslocation channel (31, 38). However, despite analogous functional and structural properties, flagellin and T3SS inner rod proteins share little primary amino acid sequence similarity. The question of how NLRC4 could respond to structurally diverse ligands has recently been resolved by the discovery that cytosolic recognition of flagellin and inner rod proteins is mediated not directly by NLRC4 but instead by the NAIP subfamily of NLRs (32, 37). According to the current model, NAIP5 and NAIP6 directly recognize flagellin, whereas NAIP2 recognizes T3SS rod proteins (Figure 3). Epistasis analysis indicates that activated NAIPs induce downstream oligomerization and activation of NLRC4 (37), which appear to be required for the downstream recruitment and activation of CASP1 by NLRC4. In addition, CASP1 activation by mouse NLRC4 requires phosphorylation of NLRC4 on serine 533. PKCδ may be the kinase that phosphorylates NLRC4, but other IY31CH04-Vance ARI 24 February 2013 11:58 Phagosome S. Typhimurium L. pneumophila T4SS IL-1β/IL-18, pyroptosis CASP1 NAIP5/6 NLRC4 NAIP5/6 CASP1 processing ASC (adaptor) CASP1 P. aeruginosa SS Pyroptosis S. flexneri T3 NAIP2 NLRC4 NAIP2 = Flagellin = T3SS rod T3SS Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. Pyroptosis T3SS Figure 3 Activation of NAIP/NLRC4 inflammasomes by bacteria. NAIP/NLRC4 inflammasomes are activated following detection of flagellin or T3SS rod proteins secreted into the host cytosol during bacterial infection by Legionella pneumophila, Salmonella enterica serovar Typhimurium, Shigella flexneri, and Pseudomonas aeruginosa. Ligand specificity is determined by the NAIP proteins: NAIP5 and NAIP6 recognize flagellin, and NAIP2 recognizes the T3SS rod. The CARD-containing NLRC4 serves as an adaptor for recruitment of CASP1 downstream of the NAIPs. Phosphorylation of NLRC4 is required for NLRC4 function. NAIP, NLRC4, and CASP1 are sufficient to initiate pyroptosis, but processing of the IL-1β and IL-18 cytokines requires recruitment of the ASC adaptor into the complex. Abbreviations: T3SS, type III secretion system; T4SS, type IV secretion system; CARD, caspase activation and recruitment domain. kinases may also be involved (39). It remains unclear how NLRC4 kinases are activated and whether this occurs downstream of ligand recognition by NAIPs. NAIPs. Naip5 was originally identified as a gene that mediates resistance of B6 mouse macrophages to intracellular replication of L. pneumophila (40, 41). By contrast, macrophages from the A/J mouse strain carry a partially defective Naip5 allele and are thus highly permissive to L. pneumophila replication. Naip5 is one of several tandemly repeated Naip genes in mice, but Naip5 is the only Naip gene essential for the restriction of L. pneumophila replication, despite the expression in B6 mice of functional Naip1, 2, 5, and 6 transcripts (40, 41). Among different inbred mouse strains, the Naip locus contains variable numbers of Naip paralogs. Individual Naip genes also vary from strain to strain; for example, the A/J Naip5 allele encodes 14 amino acid polymorphisms compared with the B6 allele and appears to be expressed at lower levels (41). However, the precise polymorphism(s) in Naip5 responsible for the permissiveness of A/J remains unknown (42). The requirement for Naip5 in restriction of L. pneumophila replication was confirmed by the generation of Naip5−/− mice on the B6 background (27). NAIP proteins contain a central NBD and C-terminal LRRs, but they differ from other NLRs in that they contain three N-terminal baculovirus inhibitor-of-apoptosis repeats (BIRs). The BIRs appear critical for NAIPs to activate NLRC4 (37), but the mechanism of www.annualreviews.org • Recognition of Bacteria by Inflammasomes BIR: baculovirus inhibitor-of-apoptosis repeat 77 IY31CH04-Vance ARI 24 February 2013 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. NLRP: NLR family, PYRIN domain– containing (formerly known as NALP) 11:58 activation is completely unknown. Consistent with the proposed role of the NBD in mediating protein-protein oligomerization, mutation of the NBD abolishes the ability of NAIP5 to induce NLRC4 oligomerization (37). The LRRs of NAIPs appear to be responsible for ligand recognition (E. Kofoed, J. Tenthorey, and R. Vance, unpublished observations). Physical association of bacterial flagellin to NAIP5 and NAIP6 (but not NAIP2) has been shown recently using a variety of biochemical approaches (32, 37). Similarly, T3SS inner rod proteins associate with NAIP2 (but not NAIP5). These biochemical studies were supported by knockout and knockdown studies. For example, Naip5−/− macrophages exhibit a selective defect in the response to flagellin but respond normally to inner rod proteins (37, 43). Conversely, knockdown of Naip2 selectively affects NLRC4 activation in response to inner rod proteins but does not affect flagellin recognition (32, 37). Taken together, these observations suggest a receptor-ligand model for activation of the NAIP/NLRC4 inflammasomes (Figure 3) but do not rule out the involvement of additional proteins. Human NAIP. In humans, L. pneumophila is the causative agent of a severe pneumonia known as Legionnaires’ disease (44). Human alveolar macrophages and monocytes cultured ex vivo generally support robust L. pneumophila replication, which contrasts with the restricted replication seen in most mouse macrophages (45, 46). This difference between human and mouse macrophages is likely explained by the recent report that human NAIP (hNAIP) does not respond to flagellin or T3SS inner rod proteins but instead responds specifically to the T3SS needle protein from Chromobacterium violaceum (32). Because C. violaceum is not a significant human pathogen, it is important that T3SS needle proteins from a variety of clinically important pathogens, such as Shigella, Salmonella, and Burkholderia, are also detected by hNAIP (32). Whether hNAIP detection of T3SS needle proteins is relevant during infection by these 78 von Moltke et al. or other common bacterial pathogens warrants further study. It is striking that the needle proteins recognized by hNAIP are, like the mouse NAIP ligands, α-helical proteins that oligomerize to form a protein-translocation channel. Because these ligands share little in the way of primary sequence, a tempting hypothesis is that all NAIP ligands share a unique secondary structural characteristic that renders them suitable as targets of innate immune recognition. Unlike the tandemly duplicated arrays of Naip genes in mice, the human NAIP gene locus is believed to harbor only a single full-length functional ortholog; however, copy number variation has been reported between geographic human populations (47, 48). It is therefore possible that in humans, as in mice, NAIP function is variable among individuals. The NLRP1 Inflammasome NLRP1 was a key constituent of the originally identified inflammasome (1), but the physiological signals that activate NLRP1 were initially unknown. It has since become apparent that many mammals have NLRP1 orthologs, and some species, such as mice, harbor multiple paralogs exhibiting high allelic variation (49, 50). Here we focus on rodent NLRP1 proteins because their activation and in vivo roles are best understood. Activation of NLRP1B by anthrax lethal toxin. One of the main virulence factors of Bacillus anthracis, the causative agent of anthrax, is a bipartite protein toxin termed lethal toxin (LeTx). LeTx is composed of protective antigen (PA) and lethal factor (LF). PA forms a channel that translocates LF, a zinc metalloprotease, into the host cell cytosol (51). The best-characterized proteolytic targets of LF are the MAP kinase kinases (MAPKKs) 1–4 and 6–7. LF-mediated cleavage of MAPKKs results in their inactivation, thereby impairing numerous host defense pathways including neutrophil chemotaxis, cytokine and chemokine production, antigen presentation, and costimulation (reviewed in 52). In addition Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 to disrupting MAPKK signaling, LeTx can also cause pyroptosis of macrophages from specific strains of mice (50, 53, 54); this responsiveness to LeTx was genetically mapped to the Nlrp1b gene (50). Although NLRP1B is sufficient to confer LeTx sensitivity, its requirement was only recently verified through the creation of an Nlrp1b knockout mouse line (55). Rat macrophages also undergo pyroptosis in response to LeTx; this responsiveness was genetically mapped to a locus containing the Nlrp1 gene, suggesting that NLRP1 activation is mechanistically conserved between mice and rats (56, 57). It is worth noting that LeTx can also induce a slower cell death in murine and human macrophages that is independent of the NLRP1B inflammasome and is instead related to disruption of p38 MAP kinase signaling downstream of TLRs (58, 59). The mechanism of NLRP1B activation by LF is not fully resolved. NLRP1B activation requires the proteolytic activity of LF and is not due to recognition of LF protein alone, as catalytically dead LF does not induce pyroptosis (54, 60). As mentioned above, the known proteolytic substrates of LF are MAPKKs 1–4 and 6–7. Although plant NLRs respond to disrupted kinase signaling pathways, no strong connection between MAPKK cleavage and NLRP1B activation has been made. Recently it was shown that LF cleaves the N terminus of the responsive rat NLRP1 allele (from CDF rats) but does not cleave the nonresponsive allele (from Lewis rats). Importantly, cleavage of NLRP1 was suggested to be required for its activation, as mutations that made NLRP1 cleavage resistant abolished its ability to activate CASP1 (61). Although it is likely that the LeTx-sensitive alleles of mouse and rat NLRP1 share a conserved mechanism of activation, the site cleaved in rat NLRP1 does not appear to be conserved in any mouse NLRP1B allele. Thus it remains to be determined whether mouse NLRP1B is also directly cleaved by LF. Additionally, it is unknown whether cleavage by LF is sufficient for rat or mouse NLRP1B activation; it is possible that other LF substrates participate. Supporting this latter possibility, several groups have shown that proteasome activity is required for NLRP1B activation and that this activity is a specific requirement for activation of NLRP1B but not of NLRP3 or NLRC4 (54, 62, 63). In addition, inhibition of the N-end rule degradation pathway blocks NLRP1B activation (64). Taken together, these observations suggest a model in which LF cleaves cellular substrates that act as negative regulators of NLRP1B, and these substrates become destabilized according to the N-end rule degradation pathway (Figure 4). Human NLRP1. Human NLRP1 does not respond to LeTx, and human macrophages do not undergo pyroptosis in response to LeTx (58, 65). Use of a cell-free reconstituted system suggested that muramyl dipeptide (MDP), a fragment of bacterial peptidoglycan, might be an agonist of human NLRP1 (66). However, although MDP has been reported to activate CASP1 in human macrophages, the requirement for NLRP1 is only partial, and the effect of MDP may result primarily from its established ability to activate NOD2-dependent priming of pro-IL-1β and NLRP3 (67–69). Thus, the function of human NLRP1 remains poorly understood. A unique domain: FIIND. Both the human and mouse NLRP1 have a unique domain, termed the FIIND (domain with function to find), that is not found in other NLRs (70). Recently, investigators suggested that this domain resembles the ZU-5 and UPA domains found in proteins such as PIDD and UNC5B. In PIDD, the ZU-5/UPA domains exhibit an unusual ability to undergo autoproteolysis at a conserved HFS site (49, 71). Similar spontaneous cleavage was observed in the FIINDs of human NLRP1 and murine NLRP1B (49, 72). Mutations that inactivate this self-cleavage disrupt the ability of NLRP1B to respond to LeTx (72). Thus, self-cleavage of NLRP1B within the FIIND, which occurs independently of LeTx, appears to be a maturation step required for NLRP1B function. www.annualreviews.org • Recognition of Bacteria by Inflammasomes 79 IY31CH04-Vance ARI 24 February 2013 11:58 B. anthracis Toxin secretion PA LF TLR Toxin receptor H+ H+ H+ LF translocation into the cytosol MAPKKK Unprocessed FIIND NBD FIIND MAPKK CARD Inactive Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. LRR Autoprocessed mature form MAPK Other substrates? ? ? Cleavage of rat NLRP1 N terminus Host defense Proteasome degradation Sufficient? CASP1 Pyroptosis ASC CASP1 processing IL-1β/IL-18, pyroptosis Figure 4 Activation of the NLRP1B inflammasome by anthrax lethal toxin. Bacillus anthracis secretes lethal factor (LF) and protective antigen (PA), which together form lethal toxin (LeTx). PA binds to the toxin receptor on host cells, leading to PA processing, PA oligomerization, and binding of LF. LeTx is then endocytosed, and in an acidified endosome LF is translocated into the host cell cytosol. LF rapidly cleaves and inactivates MAP kinase kinases (MAPKKs), interfering with many host defense pathways. NLRP1 must undergo autoproteolytic processing in the FIIND domain prior to LF activation. LF cleaves the N terminus of sensitive rat NLRP1 alleles, but it is unknown whether this cleavage is sufficient for activation. As the activity of the proteasome is required for NLRP1 activation, LF may also cleave unidentified substrates that become destabilized and degraded by the proteasome and can therefore no longer act as negative regulators of NLRP1. Once NLRP1B is activated, it can recruit CASP1 directly and induce pyroptosis; upon further recruitment of ASC, the NLRP1B inflammasome can also process IL-1β and IL-18 cytokines. The NLRP3 Inflammasome NLRP3 agonists. Unlike the Nlrp1b, Nlrc4, and the Naip genes, Nlrp3 is not constitutively expressed in most resting cells. Activation of the 80 von Moltke et al. NLRP3 inflammasome therefore requires two signals: (a) NF-κB-dependent transcriptional induction downstream of TLR or NOD ligands or inflammatory cytokines and (b) an agonist Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 to induce oligomerization. The first signal, the transcriptional priming, represents a critical checkpoint: Once primed, the NLRP3 inflammasome is activated by an astonishing array of stimuli, at least in vitro. These stimuli include, but are not limited to, crystalline or particulate matter (e.g., uric acid crystals, asbestos, alum) (73–75), ATP signaling through the P2X7 receptor (76), viral infections (77), fungal products (78), bacterial RNA and DNA (79–81), and numerous bacterial toxins and effector proteins (Supplemental Table 1; see the Supplemental Material link in the online version of this article or at http://www.annualreviews.org). NLRP3 also appears to be activated in response to the gut microbiota (see below). Although structurally distinct, many of the bacterial products that activate NLRP3 (e.g., nigericin, listeriolysin O, hemolysins) share pore-forming activity, suggesting that this “pattern of pathogenesis” (82) is somehow detected by the NLRP3 inflammasome. In evaluating NLRP3 agonists, it is important to distinguish their relative contributions to priming versus oligomerization of NLRP3. In some contexts, notably human blood monocytes and THP-1 cells, secretion of autocrine ATP may cause NLRP3 to oligomerize spontaneously once primed (83). Accordingly, stimuli such as lipopolysaccharide (LPS) and MDP, which in most cells [e.g., murine macrophages (76)] merely prime NLRP3, appear sufficient for NLRP3 oligomerization in human monocytes (84, 85). Mechanism of activation. Given the variety of NLRP3 agonists, it is generally accepted that they do not act as direct ligands for NLRP3 but instead all converge on one or more disruptions of host physiology that are sensed by the NLRP3 inflammasome. A unifying mechanism for NLRP3 activation remains elusive (Figure 5). In the case of crystalline and particulate NLRP3 stimuli, the common mechanism may be lysosomal destabilization resulting in cytosolic release of lysosomal enzymes, such as cathepsin B, that somehow activate NLRP3 (86). For noncrystalline NLRP3 stimuli—including bacterial pathogens, ATP, and nigericin—guanylate-binding protein 5 (GBP5) is required in interferon (IFN)γ-primed macrophages for robust NLRP3 oligomerization, although the mechanism of GBP5 and NLRP3 interaction remains unclear (87). Importantly, NLRP3 is readily activated in the absence of IFN-γ priming; therefore, it seems GBP5 mostly functions to amplify NLRP3 signaling. Another unifying model of NLRP3 activation postulates a role for reactive oxygen species (ROS) generated by ATP signaling through the P2X7 receptor (88), by NADPHoxidase during phagocytosis of particulate stimuli (75), or by mitochondria (89, 90). How ROS might promote NLRP3 oligomerization remains unresolved. One report suggested that ROS can dissociate thioredoxininteracting protein (TXNIP) from thioredoxin, allowing TXNIP to act as a ligand for NLRP3 (91); however, more recent data demonstrating that NLRP3 signaling is unchanged in Txnip−/− macrophages strongly contradict this model (92). The ROS hypothesis also remains controversial, as ROS inhibitors may only block NLRP3 priming (93). Furthermore, macrophages deficient in NADPH-oxidase subunits respond normally to NLRP3 agonists (94–96). A model of NLRP3 ligand delivery through large membrane hemichannels formed by pannexin-1 (PANX1) (97) was similarly undermined by data from knockout mice demonstrating that Panx1−/− macrophages activate the NLRP3 inflammasome normally (98). NLRP3 activation by all agonists can be blocked by high extracellular potassium (K+ ), suggesting that efflux of intracellular K+ is a downstream convergence point (99); however, high extracellular K+ also blocks NLRP1B oligomerization (62). It seems, therefore, that additional mechanisms must exist to explain the activation of these distinct inflammasomes by distinct stimuli; the characterization of these mechanisms remains a central challenge for the field. One emerging model suggests that NLRP3 agonists converge on the mobilization of calcium (Ca2+ ), which in turn causes www.annualreviews.org • Recognition of Bacteria by Inflammasomes Supplemental Material 81 IY31CH04-Vance ARI 24 February 2013 11:58 TLR Pore-forming toxins Phagosome Nucleus Cathepsin B, etc. Nlrp3 Signal 1 ? K+ efflux? Ca2+ influx? Secreted effector ? ? ? Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. ROS ? Mitochondrion IL-1β/IL-18, pyroptosis Signal 2 Inactivated endogenous ligand? CASP1 ASC IFN-γ NLRP3 ? ? GBP5 Autoprocessing Figure 5 Activation of the NLRP3 inflammasome by bacteria. Activation of the NLPR3 inflammasome requires two signals: Transcriptional induction of Nlrp3 (Signal 1) and an agonist to drive NLRP3 oligomerization (Signal 2). The mechanism underlying the second signal remains unresolved. The model shown here postulates an endogenous ligand for NLRP3 that is activated downstream of one or more disruptions in host physiology, including generation of reactive oxygen species (ROS), potassium (K+ ) efflux, calcium (Ca2+ ) influx, and/or phagolysosomal rupture or leakage. It is unclear how bacteria cause these disruptions, although in many cases activation of NLRP3 requires secreted bacterial effectors or toxins. In IFN-γ-stimulated macrophages, guanylate-binding protein 5 (GBP5) acts to promote NLRP3 oligomerization. Because NLRP3 lacks a CARD, the adaptor ASC is required for recruitment and activation of CASP1. Abbreviation: TLR, Toll-like receptor. release of mitochondrial ligand(s) that activate NLRP3 (100). Further experiments are needed to confirm these findings and to address many remaining questions. In particular, what is the difference (if any) between NLRP3 agonists and all other signals that mobilize Ca2+ ? Although not the focus of this review, numerous noninfectious disease processes— including type II diabetes, atherosclerosis, and macular degeneration—have been linked to the NLRP3 inflammasome. Point mutations in Nlrp3 underlie the inheritable familial cold autoinflammatory syndrome, neonatal onset multisystem inflammatory disease, and Muckle- AIM2: absent in melanoma 2 82 von Moltke et al. Wells syndrome. For reviews, see References 101 and 102. The AIM2 Inflammasome Like the NLRP3 inflammasome, the AIM2 inflammasome requires transcriptional priming, but this priming occurs downstream of type I IFN signaling rather than NF-κB signaling. This pathway was first uncovered in studies of Francisella tularensis activation of CASP1 (103), which required signaling through the IFN receptor but not through any of the known NBD-LRR inflammasomes. Around the same Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 time, a DNA-sensing inflammasome was hypothesized on the basis of CASP1 activation in macrophages after double-stranded DNA (dsDNA) is delivered to the cytosol (81). These two observations were unified by the identification of AIM2 as the inflammasome that detects DNA released into the cytosol upon lysis of several pathogens, including F. tularensis (4–8). AIM2 belongs to a family of IFN-inducible PYHIN proteins that contain PYRIN and HIN-200 DNA-binding domains. Like the NLRP proteins, AIM2 includes a PYRIN domain for recruitment of CASP1 via ASC; however, AIM2 lacks an NBD, and a HIN-200 domain replaces the LRR. Thus, AIM2 is structurally distinct from the other known inflammasomes. Despite these differences, AIM2 can nonetheless orchestrate the oligomerization of a large inflammasome complex (8), and the downstream pathways (cytokine processing and pyroptosis) are indistinguishable from those of NBD-LRR proteins. Another member of the PYHIN superfamily, IFI16, has also been proposed to function in CASP1 activation in response to Kaposi’s sarcoma–associated herpesvirus (104). The generation of Aim2−/− mice confirmed that AIM2 is indeed the F. tularensis–sensing inflammasome (8, 105, 106) and that AIM2 can also detect L. monocytogenes (105, 107–110). The marked susceptibility of Aim2−/− mice to F. tularensis provides one of the best examples of the critical role inflammasomes can play in host defense against bacteria. In response to both L. monocytogenes and F. tularensis, activation of AIM2 results from bacterial lysis and release of DNA that occur upon bacterial entry into the cytosol (8, 106, 107, 111) (Figure 6). Recently, it was suggested that L. pneumophila that lacks its effector protein SdhA might also activate AIM2 via release of bacterial DNA into the host cell cytosol (112). AIM2 is the only inflammasome for which direct interaction of sensor and ligand has been visualized in an infected cell (8, 106) or analyzed crystallographically (9). The optimal ligand for AIM2 activation is dsDNA of a sufficient length (>80 bp). Modeling suggests an 80-bp dsDNA molecule could accommodate up to 20 AIM2 HIN-200 domains (9). It was therefore proposed that instead of using an NBD to mediate oligomerization and downstream signaling, AIM2 multimerizes on a DNA template. Thus, oligomerization may be a conserved principle of inflammasome signaling despite the significant differences in the protein domains found in NLR versus PYHIN inflammasomes. Other NBD-LRR Proteins Most members of the NBD-LRR protein family remain poorly characterized. NLRP12 was found to activate CASP1 even before the term inflammasome was coined (113), and polymorphisms in NLRP12, like those in NLRP3, are associated with hereditary inflammatory disorders in humans (114). Studies of Nlrp12−/− mice have uncovered diverse roles for NLRP12 in immunity and disease. Two groups described a role for NLRP12 in colonic inflammation (see below) (115, 116); additionally, in a model of contact hypersensitivity, NLRP12 appears to be required for migration of myeloid cells (117). There are also reports of a role for NLRP12 in negative regulation of NF-κB (115). Interestingly, a recent report also found a clear role for NLRP12 in immune defense against bacterial pathogens. Nlrp12−/− mice show increased bacterial load and decreased survival when infected with Yersinia pestis (118). NLRP12 appears to provide protection via induction of IL-18, which in turn induces IFN-γ; accordingly, Il18−/− and Ifnγ −/− mice are also susceptible to Y. pestis. Although the immune defect in Nlrp12−/− mice appears selective for Yersinia, it remains unclear whether NLRP12 detects the pathogen directly or by monitoring disruptions in host homeostasis. NLRP7, which is absent in mice, assembles an inflammasome in response to microbial acylated lipopeptides (119). Lastly, it should be noted that several NBD-LRR proteins (e.g., CIITA and NLRC5) seem to have diverse non-inflammasome-related functions (e.g., in regulation of gene expression). One further example may be NLRP6, which has been www.annualreviews.org • Recognition of Bacteria by Inflammasomes 83 IY31CH04-Vance ARI 24 February 2013 11:58 Phagosome L. monocytogenes F. tularensis Type I IFN Bacterial lysis Nucleus Signal 1 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. Aim2 DNA Signal 2 AIM2 ASC CASP1 IL-1β/IL-18, pyroptosis Autoprocessing Figure 6 Activation of the AIM2 inflammasome. Activation of the AIM2 inflammasome requires two signals: Transcriptional induction of Aim2 downstream of type I IFN signaling (Signal 1) and cytosolic double-stranded DNA (Signal 2). In the case of bacterial infection, e.g., by Listeria monocytogenes or Francisella tularensis, DNA is released if invading bacteria undergo lysis in the cytosol. Because AIM2 lacks a CARD, the adaptor ASC is required for recruitment and activation of CASP1. suggested to negatively regulate NF-κB and MAP kinase signaling downstream of TLR activation in a manner apparently independent of CASP1 (120). At the same time, the role of NLRP6 in regulating intestinal inflammation appears to be IL-18-, CASP1-, and ASCdependent (121, 122), suggesting that NLRP6 can form an inflammasome. Although it is surprising that NLRP6 exhibits such diverse functions, it is important not to assume that all NBD-LRR proteins act via inflammasome formation. Noncanonical Inflammasomes CASP1 is not the only inflammasome effector caspase. After confirming earlier reports (123) that the available Casp1−/− mice are actually Casp1/11−/− double knockouts, investigators showed that pyroptosis induced by Escherichia coli and Vibrio cholerae is CASP11 dependent 84 von Moltke et al. and CASP1 independent (124). Interestingly, IL-1β processing and secretion downstream of E. coli and V. cholerae require NLRP3 and CASP1 in addition to CASP11 (123, 124), suggesting that CASP11 can induce pyroptosis directly but can only induce IL-1β via downstream activation of NLRP3 and CASP1 (125, 126, 127). Surprisingly, pyroptosis induced by the noncatalytic B subunit of cholera toxin (CTB) also activates CASP11, although it is not clear how CTB would be sensed (124). One possible explanation is that CTB’s ability to activate CASP11 may result from LPS contamination of the B-subunit preparation, though the trafficking function of the B subunit may also play a role. The idea that LPS can activate CASP11 is supported by recent reports that CASP11 activation by gram negative bacteria requires TLR4, TRIF, and the type I IFN receptor Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 (125, 126). CASP11 can also be activated by purified LPS (125, 126) or type I IFN (125), and Casp11−/− mice are resistant to LPS-induced toxic shock (124, 127). Interestingly, mice deficient in both Casp1 and Casp11 were more resistant to S. Typhimurium compared with Casp1 singly deficient mice (126). As a result, Casp11-dependent pyroptosis was proposed to be effective at restricting bacterial replication only if accompanied by a Casp1-dependent recruitment of neutrophils, which eliminate extracellular bacteria. It remains unclear how CASP11 is activated. Pyroptosis downstream of known inflammasomes (NAIP/NLRC4, AIM2, NLRP1B) does not appear to require CASP11 (124, 128). Mechanistically, it is possible that a novel noncanonical inflammasome serves as a platform for CASP11 activation. Alternatively, because CASP11 expression is inducible (125–127), CASP11 may autoactivate upon expression, though one report suggests expression alone is insufficient for CASP11 activation (126). There is no direct ortholog of CASP11 in humans; instead, humans express CASP4 and CASP5 (which are absent in mice). Although CASP5 associates with the NLRP1 inflammasome in humans (1), it is not clear whether its functional role in the response to LPS in humans is similar to that of CASP11 in mice. Accumulating evidence also suggests that inflammasome complexes include more than just NLR/PYHIN proteins, ASC, and CASP1. For example, the inhibitor of apoptosis (IAP) proteins cIAP1 and cIAP2 regulate inflammasomes, although published reports disagree about whether this interaction is activating or inhibitory (129, 130). The double-stranded RNA–dependent protein kinase PKR has also been proposed to regulate multiple inflammasomes (131). EFFECTOR FUNCTIONS OF INFLAMMASOMES The development of knockout mice deficient for key inflammasome components has been critical for studying the contribution of inflammasomes to host defense and disease pathogenesis in vitro and in vivo. In some cases [notably Mycobacterium tuberculosis (132, 133)], in vitro activation of an inflammasome by a bacterial pathogen does not clearly correlate with an in vivo phenotype, but overwhelmingly, disruption of the relevant inflammasome leads to an increase in bacterial replication (colonyforming units) and a decrease in survival following bacterial infection of mice. Conversely, forced expression of inflammasome ligands or deletion of inflammasome inhibitors in bacterial pathogens that normally evade inflammasome detection leads to bacterial restriction (22, 30, 134, 135). Therefore, inflammasomes are an important component of innate immunity to bacterial pathogens, as summarized in Table 1. The control by inflammasomes of bacterial infections results from the inflammasomes’ downstream effector functions, of which pyroptosis and the processing and secretion of proIL-1β/pro-IL-18 are the best characterized. Pyroptosis Pyroptosis is thought to result from osmotic pressure generated by CASP1-dependent formation of membrane pores (136) and is characterized by the rapid release of cytosolic contents. Pyroptosis is not associated with the formation of characteristic apoptotic membrane blebs and does not require the apoptotic caspases (e.g., CASP3, 7, 9). Pyroptosis has been described in macrophages and dendritic cells but may also occur in other cell types. Surprisingly, cell lysis downstream of some NLRP3 agonists is largely undetectable or occurs hours after CASP1 autoproteolysis and IL-1β/IL-18 processing (74, 137). It is unclear why CASP1 activated via NLRP3 activates pyroptosis only inefficiently, whereas CASP1 activated downstream of NLRC4 or NLRP1B induces pyroptosis within minutes of inflammasome oligomerization in nearly all cells. The CASP1 substrate(s) required for pyroptosis remains elusive (138–140). Autoproteolysis has long been considered an essential step in activation of CASP1. Yet, www.annualreviews.org • Recognition of Bacteria by Inflammasomes 85 IY31CH04-Vance ARI 24 February 2013 11:58 Table 1 Contribution of inflammasome responses to bacterial restriction and host survivala Bacteria CASP1 ASC CFU+ CFU+ Aeromonas veronii CFU+ CFU+ Bacillus anthracis SVL− Anaplasma phagocytophilum NBD/PYHIN Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. CFU+, SVL− Burkholderia thailandensis CFU+ CFU = , SVL− CFU = /+ Chlamydia muridarum Chlamydia pneumoniae CFU+, SVL− Chlamydia trachomatis CFU = Citrobacter rodentium CFU+ Escherichia coli (O21:H+) CFU = , SVL+ Francisella tularensis CFU+, SVL− Klebsiella pneumoniae Legionella pneumophila CFU+ Listeria monocytogenes CFU = /+, SVL− Mycobacterium tuberculosis CFU = /+, SVL = /− CFU+, SVL− CFU = , SVL = /− CFU = CFU = , SVL = /− 199 Nlrp1b: CFU+, SVL− Il1R: SVL− 149, 150 Il1R: CFU+ 201 Nlrc4: CFU+, SVL− Nlrp3: CFU = , SVL− Il1R: CFU = , SVL+ Il18: CFU+, SVL− Il1β/Il18: CFU+, SVL− 153 Il1β/Il18: CFU = 30 Il1β: CFU = /+ 202 Nlrc4: CFU = Nlrp3: CFU = Salmonella enterica serovar Typhimurium CFU+, SVL− Shigella flexneri CFU+, SVL− CFU = /−, SVL = /− CFU+ Staphylococcus aureus 200 Il1R: CFU = 203, 204 Il18: CFU = 205, 206 Nlrc4: CFU+ Nlrp3: CFU+ Il1β: CFU+ Il18: CFU+ 207 Nlrc4: CFU = , SVL+ Naip5: CFU = , SVL+ Il1β: CFU = , SVL+ 196 Nlrc4: CFU = , SVL = Aim2: CFU+, SVL− Nlrc4: CFU = Nlrp3: CFU = , SVL = /− Nlrc4: CFU+ GBP5: CFU+ Nlrp6: SVL+, CFU = Nlrc4: SVL = Nlrp3: CFU = , SVL = Aim2: CFU+, SVL = Nlrc4: CFU+, SVL = Pseudomonas aeruginosa References Il18: CFU+ Bordetella pertussis Burkholderia pseudomallei Cytokines Nlrc4: CFU+ Nlrp3: CFU = Nlrc4: CFU = /+, SVL− Nlrp3: CFU = , SVL = Nlrc4; Nlrp3: CFU+ Nlrp6: CFU = Nlrp3: CFU = , SVL− 8, 106, 208 209 Il1R: CFU+ Il18: CFU = 19, 21, 30, 143, 210, 211 Il1R: CFU = , SVL = 87, 120, 135, 212, 213 Il1β: CFU+, SVL− Il1R: CFU+, SVL− Il18: CFU = , SVL = /− 132, 133, 147, 214, 215, 216 Il1R: CFU+, SVL− Il1β: CFU = /+, SVL = /− Il18: CFU+, SVL− Il1β/Il18: CFU+, SVL− 120, 157, 218–221c Il1R: CFU− Il1β: CFU+ 22,b 24, 148, 217 Il1β: CFU = Il18: CFU+ 154 Il1R: SVL = Il1β: CFU+ 222, 223 (Continued) 86 von Moltke et al. IY31CH04-Vance ARI 24 February 2013 11:58 Table 1 (Continued) Bacteria Streptococcus agalactiae (Group B) CASP1 CFU+, SVL− Streptococcus pneumoniae ASC Nlrp3: CFU+, SVL− CFU+, SVL− Nlrp3: CFU = /+, SVL− Nlrp12: CFU+, SVL− Nlrp3: CFU+, SVL− Yersinia pestis Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. Yersinia pseudotuberculosis NBD/PYHIN CFU+, SVL− CFU+ CFU+ Cytokines References 224 225, 226 118d Il1R: SVL− Il1β: SVL− Il1β/Il18: CFU+, SVL− 134e Nlrp3: CFU = a This table summarizes changes in colony-forming units (CFU) and/or host survival (SVL) from in vivo models of bacterial pathogenesis in mice deficient for inflammasome-related genes (column headings). (+) increase; (−) decrease; ( = ) no change. b CFU difference in Reference 22 seen only in the absence of ExoU. c Salmonella enterica serovar Typhimurium results vary depending on status of Nramp1 gene and route of infection (intraperitoneal versus oral). d CFU and survival differences observed only with Y. pestis expressing hexaacylated lipopolysaccharide. e CFU differences enhanced in absence of inhibitory Y. pseudotuberculosis effectors. in the absence of the adaptor protein ASC, CASP1 fails to undergo autoproteolysis (18, 25, 141) but can nevertheless induce pyroptosis in a manner requiring CASP1 protease activity (128). Induction of CASP1-dependent, ASCindependent pyroptosis occurs in response to activation of NLRC4 and NLRP1B (which contain CARDs) but not AIM2 or NLRP3 (which lack CARDs). The CARDs were proposed to mediate direct recruitment, dimerization, and activation of CASP1 without a requirement for ASC, which appears necessary only for CASP1 autoproteolysis and IL-1β/IL18 processing. In fact, pyroptosis is accelerated in the absence of ASC, suggesting an inhibitory role for this adaptor (141). The molecular mechanisms that allow uncleaved CASP1 to induce pyroptosis but not cytokine processing remain uncertain. Inflammasomes lacking CARDs (e.g., NLRP3, AIM2) require ASC for both pyroptosis and cytokine processing (4, 76). Interestingly, in many cases, innate restriction of bacterial replication is completely IL-1β/IL-18 independent. For example, replication of L. pneumophila in Il-1β/Il-18−/− bone marrow–derived macrophages is indistinguishable from that in wild-type cells (142). Similarly, L. pneumophila, Burkholde- ria thailandensis, and S. Typhimurium or L. monocytogenes overexpressing flagellin are all restricted by the inflammasome in vivo in the absence of IL-1β/IL-18 (30, 135, 143). The nature of this cytokine-independent bacterial restriction remains uncertain, but the simplest model is that pyroptotic cell death eliminates the niche that intracellular pathogens must exploit for replication (21, 144). However, studies of Casp11-deficient mice suggest that pyroptosis alone is insufficient to restrict bacterial replication and that an additional IL-1β/IL-18-independent function of CASP1 is required to recruit neutrophils that eliminate the extracellular bacteria ejected from the intracellular niche by pyroptosis (126). Cytokine Processing Despite appearing dispensable for innate restriction in some in vivo models, the CASP1dependent cytokines IL-1β and IL-18 are critical inflammasome effectors in other models, although each cytokine contributes very differently. IL-18 is known principally for its ability to induce IFN-γ production, which in turn helps to restrict intracellular pathogens. By contrast, IL-1β is critical for inflammation www.annualreviews.org • Recognition of Bacteria by Inflammasomes 87 ARI 24 February 2013 11:58 (e.g., neutrophil recruitment) and helps shape adaptive immune responses. Signaling through the widely distributed IL-1 receptor rapidly induces the expression of hundreds of inflammatory genes—including those encoding IL-6, IL-8, IL-12, COX-2, numerous chemokines, and antimicrobial peptides—as well as the IL-1β transcript itself. In the liver, IL-1β, together with IL-6, drives the acute phase response, and on CD4+ T cells, IL-1 can promote Th17 or Th2 differentiation depending on the cytokine context (145). Although IL-1β production often depends on CASP1, CASP1-independent IL-1β processing has been reported (146, 147, 148). The NLRP1B-mediated response to B. anthracis provides an interesting case study for the role of cytokines and neutrophils in bacterial restriction downstream of inflammasomes. B6 mice carrying a responsive Nlrp1b allele exhibit increased resistance to B. anthracis spore challenge compared with nonresponsive nontransgenic B6 mice, even though macrophages in the transgenic mice are susceptible to LeTxmediated lysis (149). Similar results were obtained in B6 mice congenic for a responsive allele of Nlrp1b; these mice survived spore and vegetative bacterial challenges in a manner dependent on Nlrp1b and Casp1. This restriction required the IL-1 receptor and recruitment of neutrophils to the site of infection (150), suggesting that inflammasome-dependent maturation of IL-1β is critical for restriction of B. anthracis. Therefore, although LeTx is important for B. anthracis virulence (151), likely through its inactivation of MAPKKs, the activity of LeTx is counteracted by NLRP1B-mediated detection of the toxin. Indeed, the release of intracellular pathogens by pyroptosis and the recruitment of neutrophils by IL-1β likely synergize to restrict bacterial replication in vivo. The recruitment of neutrophils also carries with it the risk of significant damage to host tissues. Therefore, in circumstances in which neutrophils fail to restrict the microbe, neutrophil recruitment is associated with severe pathology and even increased bacterial dissemination (30, 152–154). For example, Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance 88 von Moltke et al. Il1R−/− mice infected with Burkholderia pseudomallei, which can replicate in neutrophils, are protected compared with wild-type mice (153). Signaling through the IL-18 receptor induces proinflammatory cytokines and, depending on the cytokine context, can drive CD4+ T cells to Th1 and perhaps even Th2 and Th17 phenotypes (145). However, IL-18 was first identified as an IFN-γ-inducing factor, and this remains its primary contribution to innate restriction of bacterial pathogens. For example, restriction of B. pseudomallei is lost in Il18−/− mice but can be restored by exogenous IFN-γ (153). In the spleen, memory CD4+ T cells secrete IFN-γ in response to IL-18 produced by CD8α+ dendritic cells that activate the NLRC4 inflammasome in response to S. Typhimurium (155). Surprisingly, even purified flagellin can activate this response, so it was suggested that cross-presentation pathways in these CD8α+ cells deliver flagellin to the cell cytosol. The contribution of IL-1β and IL-18 to T cell differentiation and the potential adjuvanticity of endogenous factors released during pyroptosis suggest a link between inflammasome activation and the adaptive immune response. Although evidence for this link exists in influenza infections (77), relatively little work has examined the requirement of inflammasomes for adaptive immunity to bacterial pathogens. Protective immunity to Helicobacter infections requires IL-1 receptor but is independent of IL-18 (156). Conversely, mice infected with a strain of L. monocytogenes engineered to hyperinduce the NAIP5/NLRC4 inflammasome fail to develop protective immunity (135). Further work is needed to clarify how inflammasome effector functions, such as cytokine processing, regulate adaptive immune responses. Other Effector Functions Although certain inflammasomes (notably NAIP/NLRC4 and NLRP1B) can be activated within minutes without priming, IL-1β and to a lesser extent IL-18 require de novo transcription and translation before they can be processed by CASP1. Although this transcriptional priming could occur constitutively (157), some Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 data suggest that inflammasomes are connected to additional fast-acting signaling outputs. Indeed, activation of NAIP5/NLRC4 by systemic cytosolic delivery of flagellin can kill mice in less than 30 min (158). This rapid response appears not to require IL-1β/IL-18 or pyroptosis but instead depends on the biosynthesis of inflammatory signaling lipids termed eicosanoids— e.g., prostaglandins and leukotrienes—that induce vascular leakage and massive fluid loss into the gut and peritoneum (158). Of note, NAIP5/NLRC4-dependent eicosanoid biosynthesis occurs specifically in resident peritoneal macrophages and is completely absent in bone marrow–derived macrophages commonly used to study inflammasome responses (158). Interestingly, administration of purified LeTx into rats can also cause rapid (<1 h) death that is related to vascular shock and pleural (rather than peritoneal) fluid accumulation; this death appears to require NLRP1, though a role for eicosanoids is not established (56). In mice, activation of NLRP1B by purified LeTx also induces rapid (but nonlethal) vascular leakage that is ameliorated in the absence of prostaglandin biosynthesis (149, 158). During infection, localized vascular leakage resulting from inflammasome-induced eicosanoids produced by tissue-resident macrophages might provide complement, antibodies, and leukocytes access to the site of infection, though this process remains to be shown. Like the cytosolic flagellin model described above, the LPS-induced model of sepsis is also inflammasome dependent [requiring ASC, CASP11, and at some doses NLRP3 (124, 159)] but IL-1β/IL-18 independent (160). Although LPS-mediated lethality requires days, not minutes, it is interesting that lethality is delayed when prostaglandin synthesis is decreased (161). High-mobility group B1 (HMGB1) protein, which is released from cells during pyroptosis and has proinflammatory effects, has also been linked to LPS-mediated inflammation and pathology downstream of the inflammasome (160). HMGB1, IL-1β, and IL-18 are all members of a group of proteins lacking canonical secre- tion signals that rely on CASP1 for their release (162). In addition, IL-1α—a cytokine that signals through the same receptor as IL-1β and thus has essentially the same in vivo effects— lacks a signal sequence and can in some cases be secreted in a CASP1-dependent manner (163). These proteins are passively released during pyroptotic cell lysis, but there is also evidence for an active secretion process that occurs before or even in the absence of readily detectable cell lysis (74, 137). Current models for this pathway, which is calcium dependent (164–166), include microvesicle shedding and lysosome exocytosis (165–167). The relative contributions of active and passive processes to CASP1-dependent protein release likely vary by cell type, inflammasome, and agonist; it will be particularly important to investigate these processes in vivo. Another suggested effector function of CASP1 is the transcriptional induction of lipogenic genes to promote membrane biosynthesis and counteract cell death (168). This transcriptional response requires several hours and is apparently inadequate to counteract pyroptosis by CASP1, which can occur in 1–2 h. The in vivo relevance of the link between CASP1 and lipogenic genes is uncertain, and the response may be unique to the nonhematopoietic cells used in the study. In a departure from the view that pyroptosis is the dominant mechanism that restricts intracellular bacterial replication via elimination of the intracellular niche, some studies have suggested that pyroptosis-independent pathways may also restrict bacterial replication. For example, Naip5-defective A/J macrophages are highly permissive to L. pneumophila replication (see above) despite the fact that some studies (169) (but not all; see Reference 20) show normal induction of pyroptosis in A/J macrophages in response to L. pneumophila. Restriction of L. pneumophila replication in Naip5-sufficient B6 macrophages may be due to delivery of L. pneumophila phagosomes to lysosomes for degradation (19, 144, 170, 171). This activity has been reported downstream of CASP1 through its processing of CASP7 (142) and also downstream of CASP11 (172). www.annualreviews.org • Recognition of Bacteria by Inflammasomes 89 IY31CH04-Vance ARI 24 February 2013 11:58 Whether pyroptosis or altered phagosome trafficking causes bacterial restriction has been difficult to determine because there is currently no way to selectively eliminate either of these effector functions of the inflammasome. In summary, inflammasomes seem to trigger more downstream outputs than previously appreciated, although many of these outputs need further evaluation. In particular, it will be important to understand their individual contributions to host immunity and pathology in vivo. Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. INHIBITION/EVASION OF INFLAMMASOMES BY BACTERIA If an innate immune response plays an important role in host defense, then it is likely that bacterial pathogens will evolve to evade the response. The inflammasome is no exception to this general rule. L. monocytogenes and S. Typhimurium, for example, downregulate flagellin expression inside cells to evade detection by NAIP5/NLRC4 (173, 174). Enforced expression of flagellin by these bacteria in vivo results in their severe attenuation; this attenuation is reversed in Nlrc4−/− mice, demonstrating that the attenuation is indeed due to inflammasome activation (30, 135). Staphylococcus aureus meanwhile modifies its cell wall to prevent degradation by lysozymes, and this modification indirectly reduces detection by NLRP3 (175). Examples of bacterial inflammasome inhibitors include the M. tuberculosis–secreted metalloprotease Zmp1 and the P. aeruginosa– secreted phospholipase ExoU (22, 176). The various Yersinia species are perhaps best equipped with an armament of inflammasome inhibitors. Y. enterocolitica, for example, blocks CASP1 activation through the inhibitory effect of its secreted effector YopE on Rho GTPases, in particular Rac-1 (177). Which inflammasome is targeted by this activity and how endogenous Rho GTPases contribute to CASP1 activation remain unresolved. Using a YopK-deficient strain of Y. pseudotuberculosis, Brodsky et al. (134) uncovered the activation of both the NLRC4 and NLRP3 inflammasomes by the Yersinia T3SS. YopK secreted by wild90 von Moltke et al. type bacteria normally blocks this host pathway by binding the T3SS, and this inhibition is critical for host colonization. Bacterial load is attenuated in wild-type mice infected with the YopK-deficient Y. pseudotuberculosis strain compared with that in mice infected with the wildtype strain, but this attenuation is reversed in mice that are deficient for components of the inflammasome. Meanwhile, a YopJ isoform with enhanced MAP kinase and NF-κB inhibitory activity that is expressed in the Y. pestis KIM strain also leads to enhanced CASP1 activation and IL-1β secretion. Y. pestis CO92 and Y. pseudotuberculosis, in contrast, secrete a less potent YopJ isoform, suggesting another possible mechanism of inflammasome evasion (178). Lastly, a study (118) recently demonstrated that production of a nonstimulatory tetraacylated LPS allows Y. pestis to evade TLR4- and inflammasome-dependent production of protective IL-18. Evasion or inhibition of the inflammasome by bacterial pathogens means that, in many cases, inflammasome deficiency should not be expected to result in heightened susceptibility to infection. Indeed, dramatic in vivo phenotypes are not always observed in inflammasome-deficient mice (Table 1). INFLAMMASOME RECOGNITION OF THE MICROBIOTA The mammalian intestine harbors a complex microbial ecosystem, termed the microbiota, that is composed of trillions of microbes representing hundreds of species. The microbiota provides numerous benefits to its host but also poses serious challenges to the mucosal immune system in the intestinal tract. On the one hand, the immune system must detect and provide a barrier against the microbiota and incoming pathogens. On the other hand, the immune system must avoid inappropriate pathological responses to the microbiota. Disruption of the host-microbiota equilibrium can lead to severe pathology, including inflammatory bowel disease (IBD) and sepsis. The first evidence suggesting that inflammasomes may in part mediate host-microbiota Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 interactions stemmed from genome-wide association studies of IBD patients. Polymorphisms in the Nlrp3 gene have been linked to an increased risk of developing Crohn’s disease (CD), a form of IBD (179, 180). However, this link was not confirmed in a larger study of ∼13,000 CD patients, a finding that may be explained by regional differences in genetic backgrounds (181). Additionally, polymorphisms in the genes encoding IL-1β, IL-18, and IL-18 receptor accessory protein have been associated with an increased risk of developing CD (182–184). Together, these studies, and others discussed below, suggest that inflammasomes may contribute to the pathogenesis of IBD and, more broadly, may play a variety of roles in regulating homeostasis in the intestinal tract (Figure 7). The Inflammasome, Inflammation, and Tissue Repair Oral administration of dextran sulfate sodium (DSS), which causes mucus erosion and cytotoxicity to colonic epithelial cells, provides a useful model of colitis-like disease in mice. Initial studies examining a role for the inflammasome in intestinal homeostasis found that CASP1-deficient mice were less susceptible to DSS-induced colitis compared with wild-type mice, suggesting that the inflammasome contributes to disease pathogenesis (185). Consistent with this notion, administration of an IL18 neutralization antibody to wild-type mice protected against disease (185). However, more recent studies demonstrated that the inflammasome may actually provide protective responses to DSS-mediated colitis. Three groups demonstrated that mice deficient for functional CASP1 or ASC exhibit greater disease severity (186–188). Disease was associated with increased epithelial barrier permeability, resulting in increased extraintestinal dissemination of the microbiota (188). The increased barrier permeability was suggested to result from defective cell proliferation and repair of the epithelium. Il18−/− mice exhibit pathologies similar to those in Casp1−/− mice, and administra- tion of recombinant IL-18 to Casp1−/− mice reduced disease severity (187–189). Which inflammasomes control CASP1 in the context of DSS-induced colitis? Two independent studies (121, 190) reported that Nlrp3−/− mice are partially protected from DSS. By contrast, other studies have reported that Nlrp3−/− mice exhibit increased disease severity, suggesting that NLRP3 may protect against the development of DSS-induced colitis. Inconsistencies among DSS studies may result from differences in the microbiota among mouse strains and/or colonies. Hirota et al. (191) showed that Nlrp3 deficiency is associated with reduced βdefensin antimicrobial peptide production in the colon. Zaki et al. (188) demonstrated that disease in Nlrp3−/− mice is associated with increased gut barrier permeability, defective epithelial proliferation, and increased extraintestinal invasion of the microbiota. In support of a role for the microbiota in triggering disease, antibiotic administration to Nlrp3−/− mice attenuates disease progression. Thus, NLRP3 may be required for the tissue-regenerative function of IL-18 in response to DSS-induced intestinal injury. However, it remains to be shown whether the levels of colonic IL-18 in Nlrp3−/− mice are impaired and whether administration of recombinant IL-18 to Nlrp3−/− mice is protective. Additional reports have suggested that NLRP6 and NLRP12 are also important for attenuation of colonic inflammation. Impaired NLRP12 signaling in both hematopoietic and nonhematopoietic cells results in increased inflammation in the DSS model (115, 116). NLRP6-deficient mice exhibit increased intestinal permeability and inflammation and impaired IL-18 production (192, 193). Interestingly, one study (121) proposed that NLRP6 also regulates responses to DSS via regulation of the microbiota composition (see below). These alternate roles for NLRP6 are not mutually exclusive. Chronic inflammation associated with IBD is recognized as a major risk factor for the pathogenesis of colitis-associated colon cancer (CAC). As in colitis, the inflammasome reportedly plays a negative regulatory role in www.annualreviews.org • Recognition of Bacteria by Inflammasomes 91 IY31CH04-Vance ARI 24 February 2013 11:58 Mucus erosion and damaged epithelium 1 Repair Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. Lumen ? Microbial translocation 3 Mucus Regulation of microbiota composition IL-18 2 Inflammation (localized and systemic) Epithelium Lamina propria IL-1β IL-18 ? Hematopoietic cell Figure 7 Proposed roles for inflammasomes in host-microbiota interactions. ! Repair: Activation of inflammasomes by unknown signals leads to production of IL-18, which in turn leads to regeneration of epithelial cells by an unknown mechanism. Deficiencies in inflammasome signaling and IL-18 production are associated with impaired repair of the colonic epithelial barrier. " Inflammation: Disruption of the epithelial barrier and microbial translocation result in localized and systemic inflammasome activation and inflammation driven by IL-18 and IL-1β. # Regulation of the microbiota composition: Upon recognition of unknown ligands, NLRP3 and NLRP6 lead to production of IL-18, contributing to regulation of the microbiota composition through an unknown mechanism. Disruption of inflammasome signaling is associated with a dysbiotic microbiota with proinflammatory properties. the development of CAC. In a mouse model of CAC, Casp1−/− and Asc−/− mice present with increased tumor burdens and reduced levels of IL-18. Furthermore, roles for NLRP3, NLRP6, and NLRP12 in defense against CAC have also been established (186, 188, 192, 193). 92 von Moltke et al. The Inflammasome and Intestinal Dysbiosis It is now well recognized that maintaining a properly balanced microbial composition is crucial for intestinal homeostasis. Disruption of Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 this composition (dysbiosis) can result in the expansion of pathobionts—constituents of a normal microbiota that are typically harmless to the host but can become pathogenic when homeostasis is disrupted (194, 195). Recent studies have revealed complex roles for the inflammasome in regulating the microbiota composition. For example, Nlrp6−/− mice, which are more sensitive to DSS than are wild-type mice, were found by 16S rRNA deep sequencing of the intestinal microbiota to harbor an overrepresentation of bacteria from the phyla Bacteroidetes (Prevotellaceae) and TM7 (121). An expansion of these phyla was also found in Casp1−/− , Asc−/− , and Il18−/− mice but not in Nlrc4−/− , Aim2−/− and Nlrp3−/− mice (121). Dysbiosis associated with Nlrp6 deficiency appears to be transmissible (121). Wild-type mice cohoused with Nlrp6−/− mice became colonized with Prevotellaceae and TM7 and developed more severe DSS-induced colitis (121). Cohousing wild-type mice with Casp1−/− , Asc−/− , or Il18−/− mice produced similar results. Furthermore, both antibiotictreated Nlrp6−/− and wild-type mice cohoused with antibiotic-treated mutant mice exhibited reduced colitis severity that correlated with reduced levels of Prevotellaceae and TM7 (121). Colonization with these colitogenic-associated microbes correlated with increased production of intestinal CCL5 (121). Ccl5−/− mice cohoused with Nlrp6−/− mice did not become more sensitive to DSS-induced colitis despite having increased colonization of Prevotellaceae and TM7. Bone marrow chimera experiments suggested that NLRP6 and IL-18 are required in radioresistant (perhaps epithelial) cells. The authors proposed a model in which the NLRP6 inflammasome regulates the colonization of these procolitogenic-associated bacteria through a mechanism dependent on IL-18 production by intestinal epithelial cells (121). The mechanism by which IL-18 contributes to the shaping of the microbiota composition and the ligand that activates NLRP6 remain unknown. Importantly, it remains to be shown whether Prevotellaceae and TM7 are the causative agents of disease in this model. An al- ternative model is that their expansion is the indirect consequence of the host response to other members of the microbiota. In addition to producing local pathogenic effects, intestinal dysbiosis associated with impaired inflammasome signaling can trigger inflammatory disorders in distal organs. For example, the inflammasome is critical for protection against diet-induced liver inflammation, and this protection is associated with the intestinal microbiota composition (122). Asc−/− and Casp1−/− mice fed a methionine- and cholinedeficient diet (MCDD) developed more severe liver damage compared with wild-type mice, as well as enhanced hepatic steatosis and increased levels of immune cells populating the liver. Disease progression appears to depend in part on the NLRP3 inflammasome, as MCDD-fed Nlrp3−/− mice and Il18−/− mice exhibit increased liver damage and hepatic inflammation (122). Consistent with previous findings (121), Asc−/− mice also exhibited increased susceptibility to MCDD-induced liver inflammation, and this susceptibility was associated with specific alterations in the microbiota of Asc−/− mice (122). It remains to be shown whether MCDD-fed Nlrp3−/− , Nlrp6−/− , Casp1−/− , and Il18−/− mice exhibit microbiota compositions similar to those in Asc−/− mice and what microbial species are the causative agents of disease. The above studies demonstrate that the inflammasome plays an important beneficial role in controlling the microbiota composition. A recent study (196) provides evidence that inflammasomes can also mediate disease pathogenesis triggered by intestinal dysbiosis. Colony-born mice treated with a broadspectrum antibiotic cocktail followed by a DSS challenge developed a sepsis-like syndrome, rather than colitis, that was associated with extraintestinal bacterial dissemination. Antibiotic treatment induced the expansion of an antibiotic-resistant E. coli O21:H+ pathobiont in the intestinal tracts of mice. Genome sequencing of the E. coli O21:H+ isolate revealed that this strain encodes several potential virulence factors associated with septicemic clinical E. coli isolates, including a www.annualreviews.org • Recognition of Bacteria by Inflammasomes 93 ARI 24 February 2013 11:58 functional flagellin and a T3SS. The authors demonstrated that the flagellin from this E. coli can activate the NAIP5/NLRC4 inflammasome. Furthermore, mice deficient for Naip5, Nlrc4, Casp1, and Il1β function exhibited an attenuated disease phenotype when treated with antibiotics/DSS or systemically challenged with E. coli O21:H+, despite having similar levels of microbial colonization of extraintestinal tissues (196), leading to the proposal that aberrant activation of the NAIP5/NLRC4 inflammasome by E. coli O21:H+ triggers IL-1β-mediated immunopathology. Thus, taken together, the studies on recognition of the microbiota by inflammasomes indicate that inflammasomes can have both beneficial and harmful regulatory effects, and the question of how appropriate responses are maintained will be important to address in future studies. Inflammasome recognition of the microbiota remains a nascent field of research, and as such, the best practices and research tools are still emerging. One particular challenge is analysis of the complex microbiota. Although cost effective, basic laboratory culturing techniques fail to uncover this complexity, as the majority of the microbiota cannot be cultured by standard laboratory techniques. The commonly applied technique of 16S rDNA qPCR also has important caveats because 16S rDNA is a variable multicopy gene, and its abundance therefore does not correlate directly with bacterial colonization. Techniques such as microarraybased applications, 16S full-length sequencing, and pyrosequencing provide better alternative approaches to characterize the microbiota ecology with greater taxonomic resolution. Furthermore, novel culturing techniques or in vivo imaging are needed to identify how and where inflammasome activation occurs in the gut. Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance IMPLICATIONS AND PERSPECTIVE Although the diversity both of inflammasomes and of the mechanisms by which they detect and respond to infection resists simplification, we propose that inflammasomes ex94 von Moltke et al. hibit several essential properties that may account for their broad roles in host defense. First, inflammasomes exhibit the important property of autoinhibition. This prevents the unwanted initiation of inappropriate and potentially harmful inflammatory signals when no pathogen is present. For NLRs, autoinhibition is thought to be mediated by the LRR domain, which blocks NBD-mediated oligomerization through an unknown mechanism. Interestingly, a conceptually similar autoinhibitory function has been proposed for the HIN-200 domain of the non-NLR AIM2 inflammasome (9), implying that autoinhibition might be a general and essential feature of inflammasomes. Second, inflammasome activation appears highly cooperative, capable of transitioning from a fully off state to a fully on state across a narrow range of agonist concentration. This binary mode of activation contrasts with most signaling through cell surface receptors such as the TLRs, in which signaling outputs scale across a wide range of ligand concentration. The binary behavior of inflammasomes is a consequence of their highly cooperative oligomerization into a single cytosolic complex. For example, timelapse movies of fluorophore-tagged ASC strikingly illustrate its complete oligomerization within minutes (197). Functionally, cooperative inflammasome oligomerization allows for rapid and decisive high-amplitude responses; such responses may be critical in host defense. Interestingly, although the non-NLR protein AIM2 lacks an NBD, it has nevertheless been proposed to be activated by a DNA-dependent multimerization step (9), suggesting that cooperative oligomerization is a common feature of inflammasomes. Third, inflammasomes must be able to evolve rapidly to keep pace with pathogen evasion mechanisms. The LRR domain appears to be a structurally robust domain that can tolerate mutations, particularly on its concave β-sheet surface, without loss of hydrophobic core stability (198). A final unifying property of inflammasomes is that they are all localized in the host cell cytosol. This localization implies that inflammasomes will respond to pathogens, which generally access the cytosol IY31CH04-Vance ARI 24 February 2013 11:58 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. (directly or indirectly) as part of their virulence strategy, but will ignore harmless microbes, which are generally relegated to the extracellular space (82). Cytosolic localization, and the consequent ability to discriminate pathogens from nonpathogens, is a critical feature of inflammasomes that distinguishes them from TLRs, which detect extracellular-derived ligands from both pathogens and nonpathogens. Indeed, we suggest that the above properties of the NBD-LRR architecture, taken together, may explain why proteins with this architecture have been maintained alongside TLRs in animal evolution for the purposes of host defense. The unique properties of the NBD-LRR architecture may also explain why it has been deployed as the basis for much of the immune system of plants. Superimposed upon the above-mentioned core properties, inflammasomes have evolved numerous and surprisingly diverse mechanisms of activation and action, many of which we likely have yet to appreciate. FUTURE ISSUES 1. Other than IL-1β and IL-18, what are the relevant protein substrates of CASP1 that mediate pyroptosis and other potential functions of the inflammasome? 2. What are the self or microbial ligands that interact with inflammasomes? With the exception of AIM2 and the NAIPs, ligands for inflammasomes remain unknown. 3. What are the molecular mechanisms of inflammasome activation? The biochemical mechanism of activation of even the intensively studied NLRP3 inflammasome remains poorly understood. How are inflammasomes maintained in an autoinhibited state and how does ligand binding, nucleotide binding, and hydrolysis regulate inflammasome assembly? 4. Do specific cell types in vivo exhibit unique inflammasome functions? Inflammasomes have been studied primarily in hematopoietic cells (particularly monocytes and macrophages), although recent work has hinted that inflammasomes can also have important roles in nonhematopoietic cells in vivo. Even different macrophage populations can have unique inflammasome signaling outputs (e.g., eicosanoid production). How many additional functions may be discovered in vivo? 5. What microbial constituents of the microbiota are recognized by inflammasomes? How and where are they recognized? 6. Do inflammasome responses play an important role in the induction of adaptive immunity? 7. How are inflammasome responses terminated? Most studies have focused on the activation of inflammasomes, but presumably termination of inflammasome activation is also critical for the maintenance of homeostasis. DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review. ACKNOWLEDGMENTS This work was supported by investigator awards from the Cancer Research Institute (R.E.V.) and the Burroughs Wellcome Fund (R.E.V.), by a grant from the University of California Cancer www.annualreviews.org • Recognition of Bacteria by Inflammasomes 95 IY31CH04-Vance ARI 24 February 2013 11:58 Research Coordinating Committee ( J.v.M.), by a National Science Foundation Graduate Research Fellowship ( J.C.S.), by National Institutes of Health Ruth L. Kirschstein National Research Service Award Fellowship AI091068 ( J.S.A.), and by National Institutes of Health R01 grants AI063302, AI075039, and AI080749 (R.E.V.). LITERATURE CITED Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. 1. Martinon F, Burns K, Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10(2):417–26 2. Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, et al. 1992. Molecular cloning of the interleukin-1β converting enzyme. Science 256(5053):97–100 3. Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD. 1992. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356(6372):768–74 4. Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, et al. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323(5917):1057–60 5. Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, et al. 2009. An orthogonal proteomicgenomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10(3):266–72 6. Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, et al. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458(7237):514–18 7. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458(7237):509–13 8. Jones JW, Kayagaki N, Broz P, Henry T, Newton K, et al. 2010. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 107(21):9771–76 9. Jin T, Perry A, Jiang J, Smith P, Curry JA, et al. 2012. Structures of the HIN domain: DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36(4):561–71 10. Bergsbaken T, Fink SL, Cookson BT. 2009. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7(2):99–109 11. Chisholm ST, Coaker G, Day B, Staskawicz BJ. 2006. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124(4):803–14 12. Jones JD, Dangl JL. 2006. The plant immune system. Nature 444(7117):323–29 13. Ausubel FM. 2005. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6(10):973–79 14. Yue JX, Meyers BC, Chen JQ, Tian D, Yang S. 2012. Tracing the origin and evolutionary history of plant nucleotide-binding site–leucine-rich repeat (NBS-LRR) genes. New Phytol. 193(4):1049–63 15. Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. 2001. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276(30):28309–13 16. Geddes BJ, Wang L, Huang WJ, Lavellee M, Manji GA, et al. 2001. Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem. Biophys. Res. Commun. 284(1):77–82 17. Damiano JS, Stehlik C, Pio F, Godzik A, Reed JC. 2001. CLAN, a novel human CED-4-like gene. Genomics 75(1–3):77–83 18. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, et al. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430(6996):213–18 19. Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Özören N, et al. 2006. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281(46):35217–23 20. Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. 2006. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2(3):e18 21. Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, et al. 2006. The Birc1e cytosolic patternrecognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7(3):318–25 96 von Moltke et al. Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 22. Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. 2007. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204(13):3235–45 23. Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. 2008. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl. Acad. Sci. USA 105(7):2562–67 24. Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Núñez G. 2007. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur. J. Immunol. 37(11):3030–39 25. Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, et al. 2007. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 3(8):e111 26. Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Özören N, et al. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7(6):576–82 27. Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, et al. 2008. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 9(10):1171–78 28. Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, et al. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7(6):569–75 29. Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, et al. 2006. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203(4):1093–104 30. Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, et al. 2010. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11(12):1136–42 31. Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, et al. 2010. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl. Acad. Sci. USA 107(7):3076– 80 32. Zhao Y, Yang J, Shi J, Gong YN, Lu Q, et al. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477(7366):596–600 33. Garaude J, Kent A, van Rooijen N, Blander JM. 2012. Simultaneous targeting of Toll- and Nod-like receptors induces effective tumor-specific immune responses. Sci. Transl. Med. 4(120):120ra16 34. Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, et al. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA 102(26):9247–52 35. Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, et al. 2012. Structural basis of TLR5-flagellin recognition and signaling. Science 335(6070):859–64 36. Sun YH, Rolán HG, Tsolis RM. 2007. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J. Biol. Chem. 282(47):33897–901 37. Kofoed EM, Vance RE. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477(7366):592–95 38. Zhong D, Lefebre M, Kaur K, McDowell MA, Gdowski C, et al. 2012. The Salmonella type III secretion system inner rod protein PrgJ is partially folded. J. Biol. Chem. 287(30):25303–11 39. Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, et al. 2012. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490(7421):539–42 40. Diez E, Lee SH, Gauthier S, Yaraghi Z, Tremblay M, et al. 2002. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 33(1):55–60 41. Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, et al. 2003. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr. Biol. 13(1):27–36 42. Losick VP, Stephan K, Smirnova II, Isberg RR, Poltorak A. 2009. A hemidominant Naip5 allele in mouse strain MOLF/Ei-derived macrophages restricts Legionella pneumophila intracellular growth. Infect. Immun. 77(1):196–204 43. Lightfield KL, Persson J, Trinidad NJ, Brubaker SW, Kofoed EM, et al. 2011. Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect. Immun. 79(4):1606–14 44. Fields BS, Benson RF, Besser RE. 2002. Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 15(3):506–26 45. Yamamoto Y, Klein TW, Newton CA, Widen R, Friedman H. 1988. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect. Immun. 56(2):370–75 www.annualreviews.org • Recognition of Bacteria by Inflammasomes 97 ARI 24 February 2013 11:58 46. Horwitz MA, Silverstein SC. 1980. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Investig. 66(3):441–50 47. Romanish MT, Nakamura H, Lai CB, Wang Y, Mager DL. 2009. A novel protein isoform of the multicopy human NAIP gene derives from intragenic Alu SINE promoters. PLoS ONE 4(6):e5761 48. Boniotto M, Tailleux L, Lomma M, Gicquel B, Buchrieser C, et al. 2012. Population variation in NAIP functional copy number confers increased cell death upon Legionella pneumophila infection. Hum. Immunol. 73(2):196–200 49. D’Osualdo A, Weichenberger CX, Wagner RN, Godzik A, Wooley J, Reed JC. 2011. CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain. PLoS ONE 6(11):e27396 50. Boyden ED, Dietrich WF. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38(2):240–44 51. Thoren KL, Krantz BA. 2011. The unfolding story of anthrax toxin translocation. Mol. Microbiol. 80(3):588–95 52. Turk BE. 2007. Manipulation of host signalling pathways by anthrax toxins. Biochem. J. 402(3):405–17 53. Friedlander AM, Bhatnagar R, Leppla SH, Johnson L, Singh Y. 1993. Characterization of macrophage sensitivity and resistance to anthrax lethal toxin. Infect. Immun. 61(1):245–52 54. Fink SL, Bergsbaken T, Cookson BT. 2008. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. USA 105(11):4312–17 55. Kovarova M, Hesker PM, Jania L, Nguyen MT, Snouwaert JN, et al. 2012. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J. Immunol. 189(4):2006–16 56. Newman ZL, Printz MP, Liu S, Crown D, Breen L, et al. 2010. Susceptibility to anthrax lethal toxininduced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 6(5):e1000906 57. Newman ZL, Crown D, Leppla SH, Moayeri M. 2010. Anthrax lethal toxin activates the inflammasome in sensitive rat macrophages. Biochem. Biophys. Res. Commun. 398(4):785–89 58. Muehlbauer SM, Evering TH, Bonuccelli G, Squires RC, Ashton AW, et al. 2007. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle 6(6):758–66 59. Park JM, Greten FR, Li ZW, Karin M. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297(5589):2048–51 60. Klimpel KR, Arora N, Leppla SH. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13(6):1093–100 61. Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, et al. 2012. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8(3):e1002638 62. Wickliffe KE, Leppla SH, Moayeri M. 2008. Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell. Microbiol. 10(2): 332–43 63. Squires RC, Muehlbauer SM, Brojatsch J. 2007. Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J. Biol. Chem. 282(47):34260–67 64. Wickliffe KE, Leppla SH, Moayeri M. 2008. Killing of macrophages by anthrax lethal toxin: involvement of the N-end rule pathway. Cell. Microbiol. 10(6):1352–62 65. Moayeri M, Sastalla I, Leppla SH. 2012. Anthrax and the inflammasome. Microbes Infect. 14(5):392–400 66. Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, et al. 2007. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25(5):713–24 67. Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, et al. 2007. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell 129(1):45–56 68. Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, et al. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278(11):8869–72 69. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, et al. 2009. Cutting edge: NFκB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183(2):787–91 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance 98 von Moltke et al. Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 70. Tschopp J, Martinon F, Burns K. 2003. NALPs: a novel protein family involved in inflammation. Nat. Rev. Mol. Cell Biol. 4(2):95–104 71. Tinel A, Janssens S, Lippens S, Cuenin S, Logette E, et al. 2007. Autoproteolysis of PIDD marks the bifurcation between pro-death caspase-2 and pro-survival NF-κB pathway. EMBO J. 26(1):197–208 72. Frew BC, Joag VR, Mogridge J. 2012. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 8(4):e1002659 73. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440(7081):237–41 74. Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453(7198):1122–26 75. Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. 2008. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320(5876):674–77 76. Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, et al. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440(7081):228–32 77. Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206(1):79–87 78. Joly S, Sutterwala FS. 2010. Fungal pathogen recognition by the NLRP3 inflammasome. Virulence 1(4):276–80 79. Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, et al. 2011. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474(7351):385–89 80. Kanneganti TD, Özören N, Body-Malapel M, Amer A, Park JH, et al. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440(7081):233–36 81. Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, et al. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452(7183):103–7 82. Vance RE, Isberg RR, Portnoy DA. 2009. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6(1):10–21 83. Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A. 2008. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1βand IL-18 secretion in an autocrine way. Proc. Natl. Acad. Sci. USA 105(23):8067–72 84. Netea MG, Nold-Petry CA, Nold MF, Joosten L, Opitz B, et al. 2009. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113(10):2324–35 85. Martinon F, Agostini L, Meylan E, Tschopp J. 2004. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14(21):1929–34 86. Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, et al. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9(8):847–56 87. Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, et al. 2012. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336(6080):481–85 88. Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. 2007. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282(5):2871–79 89. Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, et al. 2011. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12(3):222–30 90. Zhou R, Yazdi AS, Menu P, Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–25 91. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11(2):136–40 92. Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, et al. 2010. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1βin type 2 diabetes. Nat. Immunol. 11(10):897–904 www.annualreviews.org • Recognition of Bacteria by Inflammasomes 99 ARI 24 February 2013 11:58 93. Bauernfeind F, Bartok E, Rieger A, Franchi L, Núñez G, Hornung V. 2011. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187(2):613–17 94. Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. 2010. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 116(9):1570–73 95. van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, et al. 2010. Reactive oxygen species–independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. USA 107(7):3030–33 96. van Bruggen R, Köker MY, Jansen M, van Houdt M, Roos D, et al. 2010. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood 115(26):5398–400 97. Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, et al. 2007. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26(4):433–43 98. Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, et al. 2011. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 186(11):6553–61 99. Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14(9):1583– 89 100. Murakami T, Ockinger J, Yu J, Byles V, McColl A, et al. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. J. Immunol. 109(28):11282–87 101. Strowig T, Henao-Mejia J, Elinav E, Flavell R. 2012. Inflammasomes in health and disease. Nature 481(7381):278–86 102. Martinon F, Mayor A, Tschopp J. 2009. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27:229–65 103. Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. 2007. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204(5):987–94 104. Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, et al. 2011. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 9(5):363–75 105. Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, et al. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11(5):395–402 106. Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, et al. 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11(5):385–93 107. Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. 2010. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7(5):412–19 108. Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, et al. 2010. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur. J. Immunol. 40(6):1545–51 109. Warren SE, Armstrong A, Hamilton MK, Mao DP, Leaf IA, et al. 2010. Cutting edge: Cytosolic bacterial DNA activates the inflammasome via Aim2. J. Immunol. 185(2):818–21 110. Tsuchiya K, Hara H, Kawamura I, Nomura T, Yamamoto T, et al. 2010. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J. Immunol. 185(2):1186–95 111. Peng K, Broz P, Jones J, Joubert LM, Monack D. 2011. Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell. Microbiol. 13(10):1586–600 112. Ge J, Gong YN, Xu Y, Shao F. 2012. Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking. Proc. Natl. Acad. Sci. USA 109(16):6193–98 113. Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, et al. 2002. PYPAF7, a novel PYRINcontaining Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J. Biol. Chem. 277(33):29874–80 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance 100 von Moltke et al. Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 114. Jéru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, et al. 2008. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc. Natl. Acad. Sci. USA 105(5):1614–19 115. Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, et al. 2012. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36(5):742–54 116. Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, et al. 2011. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 20(5):649–60 117. Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, et al. 2010. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J. Immunol. 185(8):4515–19 118. Vladimer GI, Weng D, Montminy Paquette SW, Vanaja SK, Rathinam VA, et al. 2012. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37(1):96–107 119. Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, et al. 2012. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36(3):464–76 120. Anand P, Subbarao Malireddi RK, Lukens JR, Vogel P, Bertin J, et al. 2012. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488(7411):389–93 121. Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, et al. 2011. NLRP6 inflammasome regulates colonic microbial ecology and for colitis. Cell 145(5):745–57 122. Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, et al. 2012. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482(7384):179–85 123. Kang SJ, Wang S, Hara H, Peterson EP, Namura S, et al. 2000. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 149(3):613–22 124. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, et al. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479(7371):117–21 125. Rathinam VAK, Kailasan Vanaja S, Waggoner L, Sokolovska A, Becker C, et al. 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150(3):606–19 126. Broz P, Ruby T, Belhocine K, Bouley D, Kayagaki N, et al. 2012. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490(7419):288–91 127. Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92(4):501–9 128. Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. 2010. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8(6):471–83 129. Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, et al. 2012. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36(2):215–27 130. Labbé K, McIntire CR, Doiron K, Leblanc PM, Saleh M. 2011. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity 35(6): 897–907 131. Lu B, Nakamura T, Inouye K, Li J, Tang Y. 2012. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488(7413):670–74 132. Walter K, Hölscher C, Tschopp J, Ehlers S. 2010. NALP3 is not necessary for early protection against experimental tuberculosis. Immunobiology 215(9–10):804–11 133. Dorhoi A, Nouailles G, Jörg S, Hagens K, Heinemann E, et al. 2012. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur. J. Immunol. 42(2):374–84 134. Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, et al. 2010. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7(5):376–87 135. Sauer JD, Pereyre S, Archer KA, Burke TP, Hanson B, et al. 2011. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc. Natl. Acad. Sci. USA 108(30):12419–24 136. Fink SL, Cookson BT. 2006. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8(11):1812–25 www.annualreviews.org • Recognition of Bacteria by Inflammasomes 101 ARI 24 February 2013 11:58 137. Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, et al. 1997. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 159(3):1451– 58 138. Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. 2007. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 282(50):36321–29 139. Agard NJ, Maltby D, Wells JA. 2010. Inflammatory stimuli regulate caspase substrate profiles. Mol. Cell. Proteomics 9(5):880–93 140. Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, et al. 2008. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol. Cell. Proteomics 7(12):2350–63 141. Case CL, Roy CR. 2011. Asc modulates the function of NLRC4 in response to infection of macrophages by Legionella pneumophila. mBio 2(4):e00117–11 142. Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, et al. 2009. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5(4):e1000361 143. Case CL, Shin S, Roy CR. 2009. Asc and Ipaf inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect. Immun. 77(5):1981–91 144. Derré I, Isberg RR. 2004. Macrophages from mice with the restrictive Lgn1 allele exhibit multifactorial resistance to Legionella pneumophila. Infect. Immun. 72(11):6221–29 145. Dinarello CA. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519–50 146. Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, et al. 2007. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130(5):918–31 147. Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, et al. 2010. Cutting edge: Caspase1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184(7):3326–30 148. Karmakar M, Sun Y, Hise AG, Rietsch A, Pearlman E. 2012. Cutting edge: IL-1β processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and CASPASE-1. J. Immunol. 189(9):4231–35 149. Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, et al. 2010. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J. Immunol. 184(1):17–20 150. Moayeri M, Crown D, Newman ZL, Okugawa S, Eckhaus M, et al. 2010. Inflammasome sensor Nlrp1bdependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 6(12):e1001222 151. Pezard C, Berche P, Mock M. 1991. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 59(10):3472–77 152. Carlsson F, Kim J, Dumitru C, Barck KH, Carano RA, et al. 2010. Host-detrimental role of Esx-1mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 6(5):e1000895 153. Ceballos-Olvera I, Sahoo M, Miller MA, del Barrio L, Re F. 2011. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1βis deleterious. PLoS Pathog. 7(12):e1002452 154. Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, et al. 2000. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12(5):581–90 155. Kupz A, Guarda G, Gebhardt T, Sander LE, Short KR, et al. 2012. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8+ T cells. Nat. Immunol. 13(2):162–69 156. Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, et al. 2012. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J. Immunol. 188(8):3594–602 157. Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, et al. 2012. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat. Immunol. 13(5):449–56 158. von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, et al. 2012. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490(7418):107–11 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance 102 von Moltke et al. Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 159. Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, et al. 2006. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24(3):317–27 160. Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, et al. 2010. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185(7):4385–92 161. Ejima K, Layne MD, Carvajal IM, Kritek PA, Baron RM, et al. 2003. Cyclooxygenase-2-deficient mice are resistant to endotoxin-induced inflammation and death. FASEB J. 17(10):1325–27 162. Keller M, Rüegg A, Werner S, Beer HD. 2008. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132(5):818–31 163. Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, et al. 2012. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36(3):388–400 164. Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, et al. 2003. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1β and IL-1α from murine macrophages. J. Immunol. 170(6):3029–36 165. MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. 2001. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity 15(5):825–35 166. Bergsbaken T, Fink SL, den Hartigh AB, Loomis WP, Cookson BT. 2011. Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J. Immunol. 187(5):2748–54 167. Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. 1999. The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell 10(5):1463–75 168. Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126(6):1135– 45 169. Lamkanfi M, Amer A, Kanneganti TD, Muñoz-Planillo R, Chen G, et al. 2007. The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J. Immunol. 178(12):8022–27 170. Fortier A, de Chastellier C, Balor S, Gros P. 2007. Birc1e/Naip5 rapidly antagonizes modulation of phagosome maturation by Legionella pneumophila. Cell. Microbiol. 9(4):910–23 171. Watarai M, Derre I, Kirby J, Growney JD, Dietrich WF, Isberg RR. 2001. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 194(8):1081–96 172. Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, et al. 2012. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37(1):35–47 173. Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47(1):103–18 174. Dons L, Rasmussen OF, Olsen JE. 1992. Cloning and characterization of a gene encoding flagellin of Listeria monocytogenes. Mol. Microbiol. 6(20):2919–29 175. Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, et al. 2010. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1β secretion. Cell Host Microbe 7(1):38–49 176. Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, et al. 2008. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 3(4):224–32 177. Schotte P, Denecker G, Van Den Broeke A, Vandenabeele P, Cornelis GR, Beyaert R. 2004. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1β. J. Biol. Chem. 279(24):25134–42 178. Zheng Y, Lilo S, Brodsky IE, Zhang Y, Medzhitov R, et al. 2011. A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages. PLoS Pathog. 7(4):e1002026 www.annualreviews.org • Recognition of Bacteria by Inflammasomes 103 ARI 24 February 2013 11:58 179. Schoultz I, Verma D, Halfvarsson J, Törkvist L, Fredrikson M, et al. 2009. Combined polymorphisms in genes encoding the inflammasome components NALP3 and CARD8 confer susceptibility to Crohn’s disease in Swedish men. Am. J. Gastroenterol. 104(5):1180–88 180. Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, et al. 2008. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat. Genet. 41(1):71–76 181. Lewis GJ, Massey DC, Zhang H, Bredin F, Tremelling M, et al. 2011. Genetic association between NLRP3 variants and Crohn’s disease does not replicate in a large UK panel. Inflamm. Bowel Dis. 17(6):1387–91 182. Nemetz A, Nosti-Escanilla MP, Molnár T, Köpe A, Kovács A, et al. 1999. IL1B gene polymorphisms influence the course and severity of inflammatory bowel disease. Immunogenetics 49(6):527–31 183. Tamura K, Fukuda Y, Sashio H, Takeda N, Bamba H, et al. 2002. IL18 polymorphism is associated with an increased risk of Crohn’s disease. J. Gastroenterol. 37(Suppl. 14):111–16 184. Zhernakova A, Festen EM, Franke L, Trynka G, van Diemen CC, et al. 2008. Genetic analysis of innate immunity in Crohn’s disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am. J. Hum. Genet. 82(5):1202–10 185. Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. 2001. IL-1β-converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl. Acad. Sci. USA 98(23):13249–54 186. Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, et al. 2010. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207(5):1045–56 187. Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, et al. 2010. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32(3):367–78 188. Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. 2010. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32(3):379–91 189. Takagi H, Kanai T, Okazawa A, Kishi Y, Sato T, et al. 2003. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand. J. Gastroenterol. 38(8):837–44 190. Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, et al. 2010. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59(9):1192–99 191. Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, et al. 2011. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm. Bowel Dis. 17(6):1359–72 192. Chen GY, Liu M, Wang F, Bertin J, Núñez G. 2011. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 186(12):7187–94 193. Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, et al. 2011. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl. Acad. Sci. USA 108(23):9601–6 194. Chow J, Mazmanian SK. 2010. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe 7:265–76 195. Round JL, Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9:313–23 196. Ayres JS, Trinidad NJ, Vance RE. 2012. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat. Med. 18(5):799–806 197. Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, et al. 2007. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14(9):1590–604 198. Bella J, Hindle KL, McEwan PA, Lovell SC. 2008. The leucine-rich repeat structure. Cell. Mol. Life Sci. 65(15):2307–33 199. Pedra JH, Sutterwala FS, Sukumaran B, Ogura Y, Qian F, et al. 2007. ASC/PYCARD and caspase-1 regulate the IL-18/IFN-γ axis during Anaplasma phagocytophilum infection. J. Immunol. 179(7):4783–91 200. McCoy AJ, Koizumi Y, Higa N, Suzuki T. 2010. Differential regulation of caspase-1 activation via NLRP3/NLRC4 inflammasomes mediated by aerolysin and type III secretion system during Aeromonas veronii infection. J. Immunol. 185(11):7077–84 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance 104 von Moltke et al. Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance ARI 24 February 2013 11:58 201. Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, et al. 2010. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J. Immunol. 185(3):1711– 19 202. Nagarajan UM, Sikes JD, Yeruva L, Prantner D. 2012. Significant role of IL-1 signaling, but limited role of inflammasome activation, in oviduct pathology during Chlamydia muridarum genital infection. J. Immunol. 188(6):2866–75 203. Shimada K, Crother TR, Karlin J, Chen S, Chiba N, et al. 2011. Caspase-1 dependent IL-1β secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS ONE 6(6):e21477 204. He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. 2010. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J. Immunol. 184(10):5743–54 205. Lu H, Yang X, Takeda K, Zhang D, Fan Y, et al. 2000. Chlamydia trachomatis mouse pneumonitis lung infection in IL-18 and IL-12 knockout mice: IL-12 is dominant over IL-18 for protective immunity. Mol. Med. 6(7):604–12 206. Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect. Immun. 76(2):515–22 207. Liu Z, Zaki MH, Vogel P, Gurung P, Finlay BB, et al. 2012. Role of inflammasomes in host defense against Citrobacter rodentium infection. J. Biol. Chem. 287(20):16955–64 208. Mariathasan S, Weiss DS, Dixit VM, Monack DM. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202(8):1043–49 209. Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, et al. 2009. NLRP3 (NALP3, cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasomedependent and -independent pathways. J. Immunol. 183(3):2008–15 210. Pereira MS, Morgantetti GF, Massis LM, Horta CV, Hori JI, Zamboni DS. 2011. Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo. J. Immunol. 187(12):6447–55 211. LeibundGut-Landmann S, Weidner K, Hilbi H, Oxenius A. 2011. Nonhematopoietic cells are key players in innate control of bacterial airway infection. J. Immunol. 186(5):3130–37 212. Tsuji NM, Tsutsui H, Seki E, Kuida K, Okamura H, et al. 2004. Roles of caspase-1 in Listeria infection in mice. Int. Immunol. 16(2):335–43 213. Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, et al. 1997. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J. Immunol. 159(7):3364–71 214. McElvania TeKippe E, Allen IC, Hulseberg PD, Sullivan JT, McCann JR, et al. 2010. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS ONE 5(8):e12320 215. Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 67(5):2585–89 216. Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, et al. 2012. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int. Immunol. 24(10):637–44 217. Schultz MJ, Rijneveld AW, Florquin S, Edwards CK, Dinarello CA, van der Poll T. 2002. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 282(2):L285–90 218. Raupach B, Peuschel SK, Monack DM, Zychlinsky A. 2006. Caspase-1-mediated activation of interleukin-1β (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 74(8):4922–26 219. Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, et al. 2006. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203(6):1407–12 220. Srinivasan A, Salazar-Gonzalez RM, Jarcho M, Sandau MM, Lefrancois L, McSorley SJ. 2007. Innate immune activation of CD4 T cells in Salmonella-infected mice is dependent on IL-18. J. Immunol. 178(10):6342–49 www.annualreviews.org • Recognition of Bacteria by Inflammasomes 105 ARI 24 February 2013 11:58 221. Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. 2010. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207(8):1745– 55 222. Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, et al. 2012. Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 205(5):807–17 223. Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, et al. 2007. Inflammasome-mediated production of IL-1β is required for neutrophil recruitment against Staphylococcus aureus in vivo. J. Immunol. 179(10):6933–42 224. Costa A, Gupta R, Signorino G, Malara A, Cardile F, et al. 2012. Activation of the NLRP3 inflammasome by group B streptococci. J. Immunol. 188(4):1953–60 225. Fang R, Tsuchiya K, Kawamura I, Shen Y, Hara H, et al. 2011. Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J. Immunol. 187(9):4890–99 226. Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, et al. 2011. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J. Immunol. 187(1):434–40 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. IY31CH04-Vance 106 von Moltke et al. IY31-Frontmatter ARI 27 February 2013 7:16 Contents Annual Review of Immunology Volume 31, 2013 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. Years in Cologne Klaus Rajewsky ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 1 The Biology of Recent Thymic Emigrants Pamela J. Fink ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !31 Immunogenic Cell Death in Cancer Therapy Guido Kroemer, Lorenzo Galluzzi, Oliver Kepp, and Laurence Zitvogel ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !51 Recognition of Bacteria by Inflammasomes Jakob von Moltke, Janelle S. Ayres, Eric M. Kofoed, Joseph Chavarrı́a-Smith, and Russell E. Vance ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !73 The Immunology of Fibrosis Georg Wick, Cecilia Grundtman, Christina Mayerl, Thomas-Florian Wimpissinger, Johann Feichtinger, Bettina Zelger, Roswitha Sgonc, and Dolores Wolfram ! ! ! ! ! ! ! ! ! 107 Memory T Cell Subsets, Migration Patterns, and Tissue Residence Scott N. Mueller, Thomas Gebhardt, Francis R. Carbone, and William R. Heath ! ! ! ! ! 137 Control of Human Viral Infections by Natural Killer Cells Stephanie Jost and Marcus Altfeld ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 163 Functional T Cell Immunodeficiencies (with T Cells Present) Luigi D. Notarangelo ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 195 Controlling Natural Killer Cell Responses: Integration of Signals for Activation and Inhibition Eric O. Long, Hun Sik Kim, Dongfang Liu, Mary E. Peterson, and Sumati Rajagopalan ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 227 Metabolic Regulation of T Lymphocytes Nancie J. MacIver, Ryan D. Michalek, and Jeffrey C. Rathmell ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 259 Mesenchymal Stem Cell: Keystone of the Hematopoietic Stem Cell Niche and a Stepping-Stone for Regenerative Medicine Paul S. Frenette, Sandra Pinho, Daniel Lucas, and Christoph Scheiermann ! ! ! ! ! ! ! ! ! ! ! 285 Interleukin-4- and Interleukin-13-Mediated Alternatively Activated Macrophages: Roles in Homeostasis and Disease Steven J. Van Dyken and Richard M. Locksley ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 317 Brain-Reactive Antibodies and Disease B. Diamond, G. Honig, S. Mader, L. Brimberg, and B.T. Volpe ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 345 v IY31-Frontmatter ARI 27 February 2013 7:16 Immunology of the Maternal-Fetal Interface Adrian Erlebacher ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 387 Regulation of Ligands for the NKG2D Activating Receptor David H. Raulet, Stephan Gasser, Benjamin G. Gowen, Weiwen Deng, and Heiyoun Jung ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 413 Pathways of Antigen Processing Janice S. Blum, Pamela A. Wearsch, and Peter Cresswell ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 443 The Immune Response in Tuberculosis Anne O’Garra, Paul S. Redford, Finlay W. McNab, and Chloe I. Bloom, Robert J. Wilkinson, and Matthew P.R. Berry ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 475 Annu. Rev. Immunol. 2013.31:73-106. Downloaded from www.annualreviews.org by University of California - Berkeley on 05/08/13. For personal use only. The Adaptable Major Histocompatibility Complex (MHC) Fold: Structure and Function of Nonclassical and MHC Class I–Like Molecules Erin J. Adams and Adrienne M. Luoma ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 529 The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting Miriam Merad, Priyanka Sathe, Julie Helft, Jennifer Miller, and Arthur Mortha ! ! ! 563 T Cell–Mediated Host Immune Defenses in the Lung Kong Chen and Jay K. Kolls ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 605 Human Hemato-Lymphoid System Mice: Current Use and Future Potential for Medicine Anthony Rongvaux, Hitoshi Takizawa, Till Strowig, Tim Willinger, Elizabeth E. Eynon, Richard A. Flavell, and Markus G. Manz ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 635 Signaling by the Phosphoinositide 3-Kinase Family in Immune Cells Klaus Okkenhaug ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 675 Broadly Neutralizing Antiviral Antibodies Davide Corti and Antonio Lanzavecchia ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 705 Molecular Control of Steady-State Dendritic Cell Maturation and Immune Homeostasis Gianna Elena Hammer and Averil Ma ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 743 Indexes Cumulative Index of Contributing Authors, Volumes 21–31 ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 793 Cumulative Index of Articles Titles, Volumes 21–31 ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 800 Errata An online log of corrections to Annual Review of Immunology articles may be found at http://immunol.annualreviews.org/errata.shtml vi Contents







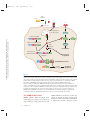

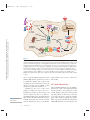









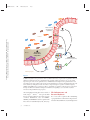



















![[Science] 10 May 2013 vol 340, issue 6133, pages 653-776](http://s1.studyres.com/store/data/004110705_1-2af1f9a9cebfa5b6ca3d0419c321be52-150x150.png)