* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download o Orbital dipole moments. Orbital precession. Spin-orbit interaction.
Survey
Document related concepts
Quantum electrodynamics wikipedia , lookup
Renormalization group wikipedia , lookup
Nitrogen-vacancy center wikipedia , lookup
Magnetic monopole wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Atomic theory wikipedia , lookup
Spin (physics) wikipedia , lookup
Magnetoreception wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron paramagnetic resonance wikipedia , lookup
Electron scattering wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Electron configuration wikipedia , lookup
Transcript
o Orbital dipole moments.
o Orbital precession.
o Spin-orbit interaction.
o Stern-Gerlach experiment.
o Total angular momentum.
o Fine structure, hyperfine structure of H and Na.
o Chapter 8 of Eisberg & Resnick
PY3P05
o Grotrian diagram for neutral sodium
showing permitted transitions, including
Na D-line transition at 589 nm.
o D-line is split into a doublet:
D1 = 589.59 nm, D2 = 588.96 nm.
o Many lines of alkali atoms are doublets.
Occur because terms (bar s-term) are
split in two.
o This fine structure can only be
understood via magnetic moments of
electron.
Na “D-line”
PY3P05
o Angular momentum for electron orbiting at distance r
and velocity v about nucleus :
Lˆ = rˆ " pˆ
o Its magnitude is therefore,
!
L = mvr
o As v = r! => L = mr2 !
o Or, in general L = I !
2
where I is the moment of inertia I = " mi ri
i
!
o
PY3P05
Electron moving with velocity (v) in a circular Bohr orbit of radius r
produces a current
i="
e
e#
="
T
2$
µl
where T is the orbital period of the electron.
o
r
Current loop!produces magnetic field, with a moment
e#
1
µl = iA = " $r 2 = " e#r 2
2$
2
L
v
e-
(1)
o
Specifies strength of magnetic dipole.
o
Magnitude of orbital angular momentum is L = mvr = m!r2.
!
e
Combining with Eqn. 1 =>
L
(2)
2m
o Electron in the first Bohr orbit with L = ! has magnetic moment
µl = "
!
µB =
!
!
e
! = 9.27x10-24 Am2
2m
Bohr Magneton
PY3P05
o
Magnetic moment can also be written in terms of the Bohr magneton:
µl =
gl µB
L
!
where gl is the orbital g-factor. Gives ratio of magnetic moment to
angular momentum (in units of !).
!
o
In vector form, Eqn 2 can be written µˆ l = "
!
o
!
o
gl µB ˆ
L
!
gl µB
l(l + 1)! = gl µB l(l + 1)
!
!
components of the angular momentum in the z-direction are
Lz = ml ! where ml = -l, -l +1, …, 0, …, +l - 1, +l.
As
L = l(l + 1)! => µl =
Magnetic moment associated with the z-component is correspondingly
!
µl z = "
gl µB
gµ
Lz = " l B ml ! = "gl µB ml
!
!
PY3P05
!
o
When magnetic moment placed in external magnetic field, it experiences a torque:
"ˆ = µˆ l # Bˆ
(3)
which tends to align dipole with field. Potential energy associated with this force is
"E = #µˆ $ Bˆ
o
Minimum potential energy occurs when µl !!B.
o
If "E = const., µl cannot align with
! B => µl precesses about B.
o
Now,
B
!
#L L sin $#%
=
= L& sin $
#t
#t
gµ
" = µl Bsin# = l B LB sin#
!
"=
and from Eqn. 3,
(4)
(5)
!
o
Equating Eqns. 4 and 5 => gl µB LBsin " = L# sin "
!
!
gµ
Larmor
=> # = l B B
frequency
!
o
Called Larmor precession. Occurs in direction of B.
!
PY3P05
o
Electron also has an intrinsic angular momentum, called spin. The
spin and its z-component obey identical relations to orbital AM:
S = s(s + 1)!
Sz = m s!
where s = 1/2 is the spin quantum number => S = 1/2(1/2 + 1)!
o
! orientations: Sz = ±1/2!
Therefore two possible
= 3 /2!
=> spin magnetic quantum number is!±1/2.
!
Follows that electron has intrinsic magnetic moments:
o
gsµB ˆ
S
!
µsz = "gsµB ms
Sˆ
µˆ s = "
!
µˆ s
where gs (=2) is the spin g-factor.
!
!
PY3P05
o
Confirmed quantisation of electron spin into
two orientations.
o
Potential energy of electron spin magnetic
moment in magnetic field in z-direction is
"E = #µˆ s $ Bˆ = #µsz B
= gsµB msB
o
The resultant force is
! Fz = "
o
dB
d(#E)
= "µB gsms z
dz
dz
As gsms = ±1,
!
o
Fz = ±µB
dBz
dz
The deflection distance is then,
2
F "L%
µ L2 dBz
!
z = 1/2at 2 = 1/2 $ ' = ± B
m #v &
4KE dz
PY3P05
!
o Conclusion of Stern-Gerlach experiment:
o With field on, classically expect random distribution at target. In fact find two
bands as beam is split in two.
o There is directional quantisation, parallel or antiparallel to B.
o Atomic magnetic moment has µz = ±µB.
o Find same deflection for all atoms which have an s electron in the outermost
orbital => all angular momenta and magnetic moments of all inner electrons
cancel. Therefore only measure properties of outer s electron.
o The s electron has orbital angular momentum l = 0 => only observe spin.
PY3P05
o Experiment was confirmed using:
Element
H
Na
K
Cu
Ag
Cs
Au
Electronic Configuration
1s1
{1s22s22p6}3s1
{1s22s22p63s23p6}4s1
{1s22s22p63s23p63d10}4s1
{1s22s22p63s23p63d104s24p64d10}5s1
{[Ag]5s25p6}6s1
{[Cs]5d104f14}6s1
o In all cases, l = 0 and s = 1/2.
o Note, shell penetration is not shown above.
PY3P05
o Fine-structure in atomic spectra cannot be explained by Coulomb interaction
between nucleus and electron.
o Instead, must consider magnetic interaction between orbital magnetic moment and
the intrinsic spin magnetic moment.
o Called spin-orbit interaction.
o Weak in one-electron atoms, but strong in multi-electron atoms where total orbital
magnetic moment is large.
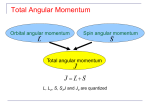
o Coupling of spin and orbital AM yields a total angular momentum, Jˆ.
!
PY3P05
o
v
Consider reference frame of electron: nucleus moves about
electron. Electron therefore in current loop which produces
magnetic field. Charged nucleus moving with v produces a
current:
r
+Ze
-e
ˆj = "Zevˆ
o
o
o
According to Ampere’s Law, this produces a magnetic field,
which at electron is
ˆ
ˆ = µ0 j # rˆ = $Zeµ0 vˆ # rˆ
B
!
4" r 3
4"
r3
Ze rˆ
Using Coulomb’s Law: Eˆ =
4 "#0 r 3
!
1
=> Bˆ = " 2 vˆ # Eˆ
(5)
c
!
where c = 1/ "0µ0
! is the magnetic field experienced by electron through E
This
! exerted on it by nucleus.
r
+Ze
-e
v
B
j
PY3P05
We know that the orientation potential energy of magnetic dipole moment is "E = #µˆ s $ Bˆ
o
but as µˆ s = " gsµB Sˆ => #E = gsµB Sˆ $ Bˆ
!
!
!
Transforming back to reference frame with nucleus, must include the factor of 2 due to
Thomas precession (Appendix O of Eisberg & Resnick):
o
!
"E so =
1 gsµB ˆ ˆ
S#B
2 !
(6)
o
This is the spin-orbit interaction energy.
o
More convenient to express
! in terms of S and L. As force on electron is
dV (r) rˆ
1 dV (r) rˆ
Fˆ = "eEˆ = "
=> Eˆ =
dr r
e dr r
can write Eqn. 5 as
1 1 dV (r)
Bˆ = " 2
vˆ # rˆ
ec r dr
!
PY3P05
!
Lˆ = rˆ " mvˆ = #mvˆ " rˆ => Bˆ =
1 1 dV (r) ˆ
L
emc 2 r dr
o
As
o
Substituting the last expression for B into Eqn. 6 gives:
!o Evaluating g and µ , we obtain:
s
B
o
For hydrogenic atoms,
V (r) = "
!
o
"E so =
gsµB 1 dV (r) ˆ ˆ
S#L
2emc 2 ! r dr
1 1 dV (r) ˆ ˆ
S#L
2m 2c 2 r dr
General
form
Ze 2
dV (r)! Ze 2
=>
=
4 #$0 r
dr
4 #$0 r 2
Substituting into equation for "E:
"E so =
1 1 Ze 2 ˆ ˆ
S%L
2m 2c 2 r 4 #$0 r 2
=
e2
Z! ˆ ˆ
S%L
4 #$0 !c 2m 2cr 3
!
"E so = #
o
"E so =
Z! ˆ ˆ
S$L
2m 2cr 3
Hydrogenic
form
(7)
!
Expression for spin-orbit interaction in terms of L and S. Note, " = e 2 /4 #$0 !c is the fine
structure constant.
!
!
PY3P05
o
Transition which gives rise to the Na D-line
doublet is 3p#3s.
o
3p level is split into states with total angular
momentum j=3/2 and j=1/2, where j = l ± s.
o
In the presence of additional externally magnetic
field, these levels are further split (Zeeman
effect).
o
Magnitude of the spin-orbit interaction can be
calculated using Eqn. 7. In the case of the Na
doublet, difference in energy between the 3p3/2
and 3p1/2 sublevels is:
"E = 0.0021 eV (or 0.597 nm)
PY3P05
o
Spectral lines of H found to be composed of
closely spaced doublets. Splitting is due to
interactions between electron spin S and the
orbital angular momentum L => spin-orbit
coupling.
o
H# line is single line according to the Bohr or
Schrödinger theory. Occurs at 656.47 nm for
H and 656.29 nm for D (isotope shift, "$~0.2
nm).
o
H#
Spin-orbit coupling produces fine-structure
splitting of ~0.016 nm. Corresponds to an
internal magnetic field on the electron of about
0.4 Tesla.
PY3P05
o
z
Orbital and spin angular momenta couple together via the spinorbit interaction.
Sˆ
Jˆ
o
Internal magnetic field produces torque which results in
precession of Lˆ and Sˆabout their sum, the total angular
momentum:
Jˆ = Lˆ + Sˆ
o
!
!
! L-S coupling
!
Called
or Russell-Saunders coupling. Maintains
fixed magnitude and z-components, specified by two quantum
numbers j and
!mj:
J = j( j + 1)!
Lˆ
Vector model
of atom
!
Jz = m j !
where mj = -j, -j + 1, … , +j - 1, +j.
o
! values of j? Must use vector inequality
But what are the
| Lˆ + Sˆ |"|| Lˆ | # | Sˆ ||
=>| Jˆ |"|| Lˆ | # | Sˆ ||
PY3P05
!
o
From the previous page, we can therefore write
j( j + l)! "| l(l + l)! # s(s + l)! |
o
o
Since, s = 1/2, there are generally two members of series that satisfy this inequality:
j = l + 1/2, l - 1/2
For l = 0 => j = 1/2
!
o
Some examples vector addition rules
o J = L + S, L = 3, S = 1
L + S = 4, |L - S| = 2, therefore J = 4, 3, 2.
o L = l1 + l2, l1 = 2, l2 = 0
l1 + l2 = 2, | l1 - l2 | = 2, therefore L = 2
o J = j1 + j2 , j1 = 5/2, j2 = 3/2
j1 + j2 = 4, | j1 - j2 | = 1, therefore J = 4, 3, 2, 1
PY3P05
o For multi-electron atoms where the spin-orbit
coupling is weak, it can be presumed that the
orbital angular momenta of the individual
electrons add to form a resultant orbital angular
momentum L.
o This kind of coupling is called L-S coupling or
Russell-Saunders coupling.
o Found to give good agreement with observed
spectral details for many light atoms.
o For heavier atoms, another coupling scheme
called j-j coupling provides better agreement
with experiment.
PY3P05
o “L-S coupling” breaks down for heavier elements when spin-orbit interaction
energy become greater that nuclear-electron interaction.
o For elements with larger nuclear charge (e.g., Z=50), spin-orbit interactions become
as strong as the interactions between individual spins or orbital angular momenta.
% spin and orbital angular momenta of individual electrons tend to couple to form
individual electron angular momenta.
o Therefore write
J1 = L1 + S1
J2 = L2 + S2
etc
o Total angular momentum is then J = " J i
i
!
PY3P05
o Total angular momentum can be visualised as
precessing about any externally applied magnetic
field.
o Magnetic energy contribution is proportional Jz.
o Jz is quantized in values one unit apart, so for the
upper level of the sodium doublet with j=3/2, the
vector model gives the splitting in bottom figure.
o This treatment of the angular momentum is
appropriate for weak external magnetic fields where
the coupling between the spin and orbital angular
momenta can be presumed to be stronger than the
coupling to the external field.
PY3P05













![NAME: Quiz #5: Phys142 1. [4pts] Find the resulting current through](http://s1.studyres.com/store/data/006404813_1-90fcf53f79a7b619eafe061618bfacc1-150x150.png)