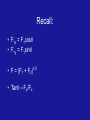* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1. Electrostatics
Negative mass wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Mass versus weight wikipedia , lookup
Elementary particle wikipedia , lookup
Magnetic monopole wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Work (physics) wikipedia , lookup
Maxwell's equations wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Speed of gravity wikipedia , lookup
Weightlessness wikipedia , lookup
Electromagnetism wikipedia , lookup
Field (physics) wikipedia , lookup
Fundamental interaction wikipedia , lookup
Anti-gravity wikipedia , lookup
Atomic nucleus wikipedia , lookup
Lorentz force wikipedia , lookup
Electricity Charge and Field Presentation 2003 R. McDermott Static Electricity • • • • • Non-moving charges. Characteristic of insulators. Positive and negative charges. Protons in a solid can’t move. Changes in charge are due to electron transfer. • Friction produces static electricity. Two Kinds of Charge • • • • Unlike charges attract Like charges repel Both charges attract neutral objects Removing electrons results in positive charge (silk on glass). • Adding electrons results in negative charge (fur on plastic). Conservation of Charge • Total charge is constant. • Electrons transfer from more negative to less negative object. • Most everyday objects are neutral (most atoms are neutral). • Identical objects in contact develop the same charge. Atoms Contain Charge • Protons inside nucleus are positive. • Electrons outside nucleus are negative. • Proton and electron have same charge magnitude. • Electrons can move within solid material The Smallest Charge • The charge on an electron (-1), or proton (+1) is one “elementary” charge. • Which equals 1.602 x 10-19 Coulombs • Charge is quantized • Discrete amounts only • 1e, 2e, 3e, 4e etc • Quarks of sub-atomic physics have -1/3 and +2/3 charge Producing Localized Charges • Rubbing • Inducing a nonsymmetrical charge distribution. • Conduction • Grounding (Induction) – Connect with wire leading to ground Inducing a nonsymmetrical charge distribution. In the picture to the right, a negatively charged rod brought close causes electrons in the object to move away (downward). Even though the object is still neutral, its top is now slightly positive, and its leaves slightly negative as a result. Inducing a nonsymmetrical charge distribution. In the picture to the right, a positively charged rod brought close causes electrons in the object to move toward it (upward). Even though the object is still neutral, its top is now slightly negative, and its leaves slightly positive as a result. Charging by Conduction • An object is charged by conduction if you touch a charged object to it, allowing electrons to be transferred. • Using a negative object results in a negative charge. Using a positive object results in a positive charge, as shown to the right: Charging by Induction • An object is charged by induction if you touch a neutral object (ground) to it, while holding a charged object nearby. • Using a negative object results in a positive charge. Using a positive object results in a negative charge, as shown to the right: Electroscope • Detects charge with leaves that repel or needle that rotates. Charging by Induction Charging by Induction Insulators and Conductors • Conductors carry charge easily – Metals have many free electrons – Ionic liquids – Plasmas • Insulators conduct poorly – Dry gases – Wood, paper, cloth, glass, etc • Semiconductors (silicon, germanium) are insulators that can be altered to conduct. Coulomb’s Law • Electric force is proportional to product of charges divided by square of distance between them • Q in coulombs • k is Coulomb constant • k = 8.988x109 Nm2/C2 • constant e0 is the permittivity of free space • Applies to point charges Example 1 – Calculate Coulomb Force • Find the force between two objects with charge 1 Coulomb at a separation of one meter. Ans. 9x109 N • If their mass is 1 kg each, what is the gravitational force between Ans. 6.7x10-11 N them? • How do the two forces compare? Example 2 • Find force between two, 1 -coulomb (10-6 coulomb) charges at separation of 20 cm F = kQ1Q2/r2 F = (9x109 N-m2/C2)(1x10-6 C)(1x10-6 C)/(0.20 m)2 F = 0.225 N Electrostatic Force and Vectors • Fnet = F1 + F2 + F3 + … • Principle of superposition of forces • Use component method of vector addition • Fx = F1x + F2x Fy = F1y + F2y Recall: • F1x = F1cosq • F1y = F1sinq • F = (F1 + F2)1/2 • Tanq = Fy/Fx Electric Field • Force acting at a distance vs. field concept. • Field, E, at a point is the force on a positive charge at that point divided by magnitude of that charge. • Direction is the same as the direction of the force on a positive charge. • The spacing of field lines indicates the strength of E. Electric Field Strength • Units: newtons per coulomb • Defined as E = F/q • E = kQ/r2 is the field strength due to a point charge Q as measured at some point ‘r’ from Q. Problem Solving • Draw careful diagrams • Apply Coulomb’s Law to get magnitude of forces or fields • Determine direction of forces by considering like and unlike charges • Show and label each vector force or field • Add vectorially to get resultant • Use symmetry when possible Three Charges in a Line • Each charge will experience two electric fields and therefore two forces; one from each of the other two charges. • The net field (and force) will be the vector sum of those two fields (and forces). Field due to 2 Like Charges Field due to Unlike Charges Fields and Conductors • • • • Field inside conductor is zero. If not, force F=qE would make charges move. Charge spreads out optimally on surface. Charge +Q inside spherical uncharged shell induces -Q on inside surface of shell. • +Q then exists on outside surface of shell. • Electric field just outside a conductor is always perpendicular to the surface. Acknowledgements • • • • Zoomschool.com Glenbrook South Physics Fizzics Fizzle at Thinkquest.com Dr. Philip M. Dauber